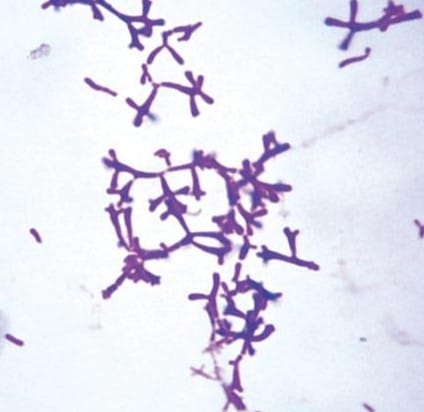
The MicroByte Series - The True Bifid: Bifidobacterium adolescentis?
History
Have you ever taken probiotics?
If so, have you ever checked the label to see which kind of bacteria are present within your probiotic capsule?
If you have, you may have noticed that along with well known Lactobacillus, Bifidobacterium often makes an appearance. Sometimes, it is a part of a blend with other beneficial bacteria, and other times it takes centre stage of its own. The many health benefits we gain from Bifidobacterium-based probiotics are, thanks to the work of Dr. Henry Tissier.
In 1899, paediatrician Henry Tissier was the first to observe a Y- or bifid-shaped microorganism in the faeces of breastfed infants. Due to its unique shape, he named it Bacillus bifidus communis. He also noticed that this bacterium was nearly absent in the faeces of infants with diarrhoea. Based on these observations, he suggested that supplementing such beneficial bacteria could help restore a healthy gut in infants suffering from diarrhoea. The bacterium was later reclassified under the genus Bifidobacterium by Danish microbiologist Orla-Jensen in 1924. Ever since, there has been extensive research exploring the role of Bifidobacterium species in promoting infant and adult health.
Recent studies support Dr. Henry Tissier’s findings, revealing that approximately 95% of the gut microbiota in healthy, breastfed infants is composed of Bifidobacterium. Although this proportion naturally declines with age, the presence of these bacteria continues to be associated with a wide range of health benefits.
Within the Bifidobacterium genus, Bifidobacterium adolescentis is one species that is found in lower numbers in infants and children compared to other bifidobacterial species. However, it becomes one of the most prominent species in adults, making up approximately 5% of the total faecal bacteria in healthy adults. Interestingly, although its overall abundance may decline with age, Bifidobacterium adolescentis tends to persist longer in the gut in older adults.
Health benefits
Bifidobacterium adolescentis, a common adult gut bacteria plays an important role in maintaining overall health. Being a butyrate producer it is linked to numerous health benefits. Studies show a correlation of low Bifidobacterium adolescentis with metabolic diseases like obesity and diabetes. One of the rat model studies showed that there was an increase in the number of Bifidobacterium adolescentis after the weight loss indicating that obesity has a role in regulating the number of Bifidobacterium adolescentis. Another study showed that Bifidobacterium adolescentis harboured a metabolic gene responsible for blood-sugar regulation, that might confer anti-diabetic effects. It is one of the major producers of Gamma-aminobutyric acid (GABA), a neurotransmitter, responsible for calming effect. This species reduces pro-inflammatory cytokine levels and recruits beneficial immune cells, thereby mitigating chronic inflammation and enhancing gut barrier integrity. These effects are associated with improved fracture healing, as reduced systemic inflammation and strengthened gut barrier function contribute to better recovery outcomes.
Applications
The food industry is trying its best to innovatively add the strains of Bifidobacterium adolescentis into various food products. Bifidobacterium adolescentis along with other probiotics are added to low-fat synbiotic cream cheese formulated with herbal gums which is targeted for adult gut health. The high GABA producing Bifidobacterium adolescentis strains are also used in probiotic milk beverages. Apart from the food industry, pharmaceuticals are also interested in the production of probiotic formulations for better health outcomes. Several studies have suggested Bifidobacterium adolescentis as a possible ‘psychobiotic’ to treat mental health and sleep disorders along with gut health and metabolic syndrome.
Fun Facts
Not all Bifidobacterium are truly "bifid"! Most species in this genus are naturally rod-shaped and only take on a Y-shape (bifid form) under stress. But Bifidobacterium adolescentis? It’s naturally bifid!
In fact, it can even change its shape based on its environment. When traveling through the small intestine’s higher pH, it transforms itself into a sphere (coccus) shape to roll along more easily. Once it reaches the colon’s slightly acidic pH, it reverts to its Y-shape to anchor itself effectively.
Do you think Bifidobacterium adolescentis might be the only one truly living up to the name ‘Bifidobacterium’?!
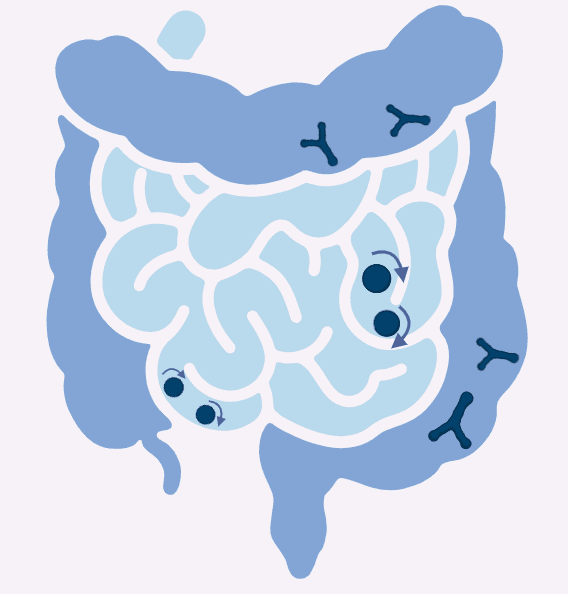
Transition of Bifidobacterium adolescentis from a spherical shape in the high pH of the small intestine to a Y-shape in the acidic colon to aid movement and anchoring.
Microbe Profile
Shape : Branched morphology- Y shape
Gram nature: Gram Positive
Spore formation : No
Biofilm formation : Weak Biofilm former
Oxygen requirement :Obligate Anaerobe-Thrives in the absence of oxygen
Optimal temperature :37°C
Optimal pH : 5.5
Nutrient usage : Galactose, Lactose, Galactooligosaccharides
Taxonomic Classification
Domain: Bacteria
Phylum: Actinomycetota (or Actinobacteria)
Class: Actinomycetia
Order: Bifidobacteriales
Family: Bifidobacteriaceae,
Genus: Bifidobacterium
Species: Bifidobacterium adolescentis
-Khushi.C
References
Qian, X., Si, Q., Lin, G., Zhu, M., Lu, J., Zhang, H., Wang, G., & Chen, W. (2022). Bifidobacterium adolescentis Is Effective in Relieving Type 2 Diabetes and May Be Related to Its Dominant Core Genome and Gut Microbiota Modulation Capacity. Nutrients, 14(12), 2479. https://doi.org/10.3390/nu14122479
Duranti, S., Ruiz, L., Lugli, G.A. et al. Bifidobacterium adolescentis as a key member of the human gut microbiota in the production of GABA. Sci Rep 10, 14112 (2020). https://doi.org/10.1038/s41598-020-70986-z
Zhang, L., Wang, P., Huang, J., Xing, Y., Wong, F. S., Suo, J., & Wen, L. (2024). Gut microbiota and therapy for obesity and type 2 diabetes. Frontiers in endocrinology, 15, 1333778. https://doi.org/10.3389/fendo.2024.1333778
Lim, S. M., & Kim, D. H. (2017). Bifidobacterium adolescentis IM38 ameliorates high-fat diet-induced colitis in mice by inhibiting NF-κB activation and lipopolysaccharide production by gut microbiota. Nutrition research (New York, N.Y.), 41, 86–96. https://doi.org/10.1016/j.nutres.2017.04.003
Roberts, J. L., Liu, G., Darby, T. M., Fernandes, L. M., Diaz-Hernandez, M. E., Jones, R. M., & Drissi, H. (2020). Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 132, 110831. https://doi.org/10.1016/j.biopha.2020.110831
Tamés, H., Sabater, C., Margolles, A., Ruiz, L., & Ruas-Madiedo, P. (2023). Production of GABA in milk fermented by Bifidobacterium adolescentis strains selected on the bases of their technological and gastrointestinal performance. Food research international (Ottawa, Ont.), 171, 113009. https://doi.org/10.1016/j.foodres.2023.113009
Shahraki, R., Elhamirad, A. H., Hesari, J., Noghabi, M. S., & Nia, A. P. (2023). A low-fat synbiotic cream cheese containing herbal gums, Bifidobacterium adolescentis and Lactobacillus rhamnosus: Physicochemical, rheological, sensory, and microstructural characterization during storage. Food science & nutrition, 11(12), 8112–8120. https://doi.org/10.1002/fsn3.3731
Murakami, H., Ko, T., Ouchi, H., Namba, T., Ebihara, S., & Kobayashi, S. (2024). Bifidobacterium adolescentis SBT2786 Improves Sleep Quality in Japanese Adults with Relatively High Levels of Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 16(11), 1702. https://doi.org/10.3390/nu16111702
Yunes, R. A., Poluektova, E. U., Vasileva, E. V., Odorskaya, M. V., Marsova, M. V., Kovalev, G. I., & Danilenko, V. N. (2020). A Multi-strain Potential Probiotic Formulation of GABA-Producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with Antidepressant Effects. Probiotics and antimicrobial proteins, 12(3), 973–979. https://doi.org/10.1007/s12602-019-09601-1
Liu, Z., Li, L., Fang, Z., Lee, Y., Zhao, J., Zhang, H., Chen, W., Li, H., & Lu, W. (2021). The biofilm-forming ability of six Bifidobacterium strains on grape seed flour. LWT, 144, 111205. https://doi.org/10.1016/j.lwt.2021.111205
Andriantsoanirina, V., Allano, S., Butel, M. J., & Aires, J. (2013). Tolerance of Bifidobacterium human isolates to bile, acid and oxygen. Anaerobe, 21, 39–42. https://doi.org/10.1016/j.anaerobe.2013.04.005
Dhanashree, Rajashekharan, S., Krishnaswamy, B., & Kammara, R. (2017). Bifid Shape Is Intrinsic to Bifidobacterium adolescentis. Frontiers in microbiology, 8, 478. https://doi.org/10.3389/fmicb.2017.00478
Amaretti, A., Bernardi, T., Tamburini, E., Zanoni, S., Lomma, M., Matteuzzi, D., & Rossi, M. (2007). Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Applied and environmental microbiology, 73(11), 3637–3644. https://doi.org/10.1128/AEM.02914-06