Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Strawberries and the Aging Gut: A Delicious Path to Microbiome Health
As the science of nutrition continues to evolve, researchers are uncovering how specific foods can nurture not just our bodies, but the communities of microbes living within us. A recent randomized controlled study published in Microbiology Spectrum explored the impact of strawberries on the gut microbiome of healthy older adults—and the results are both fascinating and promising. Over a 10-week period, 69 elderly participants were assigned to five dietary intervention groups that varied in their consumption of strawberries and capers. The group receiving the highest intake of fresh and freeze-dried strawberries, without added capers, experienced the most notable positive changes in their gut microbiota.
The researchers observed a significant increase in beta diversity, a marker of microbial variety and ecosystem stability, in those consuming more strawberries. This suggests that the fruit not only introduced beneficial compounds but also supported a broader range of gut bacteria. Importantly, there was a decrease in the abundance of microbes associated with poor health outcomes, while populations of short-chain fatty acid (SCFA) producers like Faecalibacterium and Prevotella increased. SCFAs are crucial for maintaining the gut barrier, modulating inflammation, and fueling colon cells. Furthermore, participants in the high-strawberry group showed a shift in their Firmicutes-to-Bacteroidetes ratio—a marker often linked to metabolic health—and a decline in Ruminococcaceae, a family of bacteria associated with inflammatory conditions.
These findings highlight how a simple dietary addition—rich in fiber and polyphenols like strawberries—can have meaningful effects on the gut microbiome in later life. As aging is often associated with a decline in microbial diversity and resilience, incorporating such foods may offer a tasty and natural strategy to support gut health, reduce inflammation, and promote healthy aging from within.
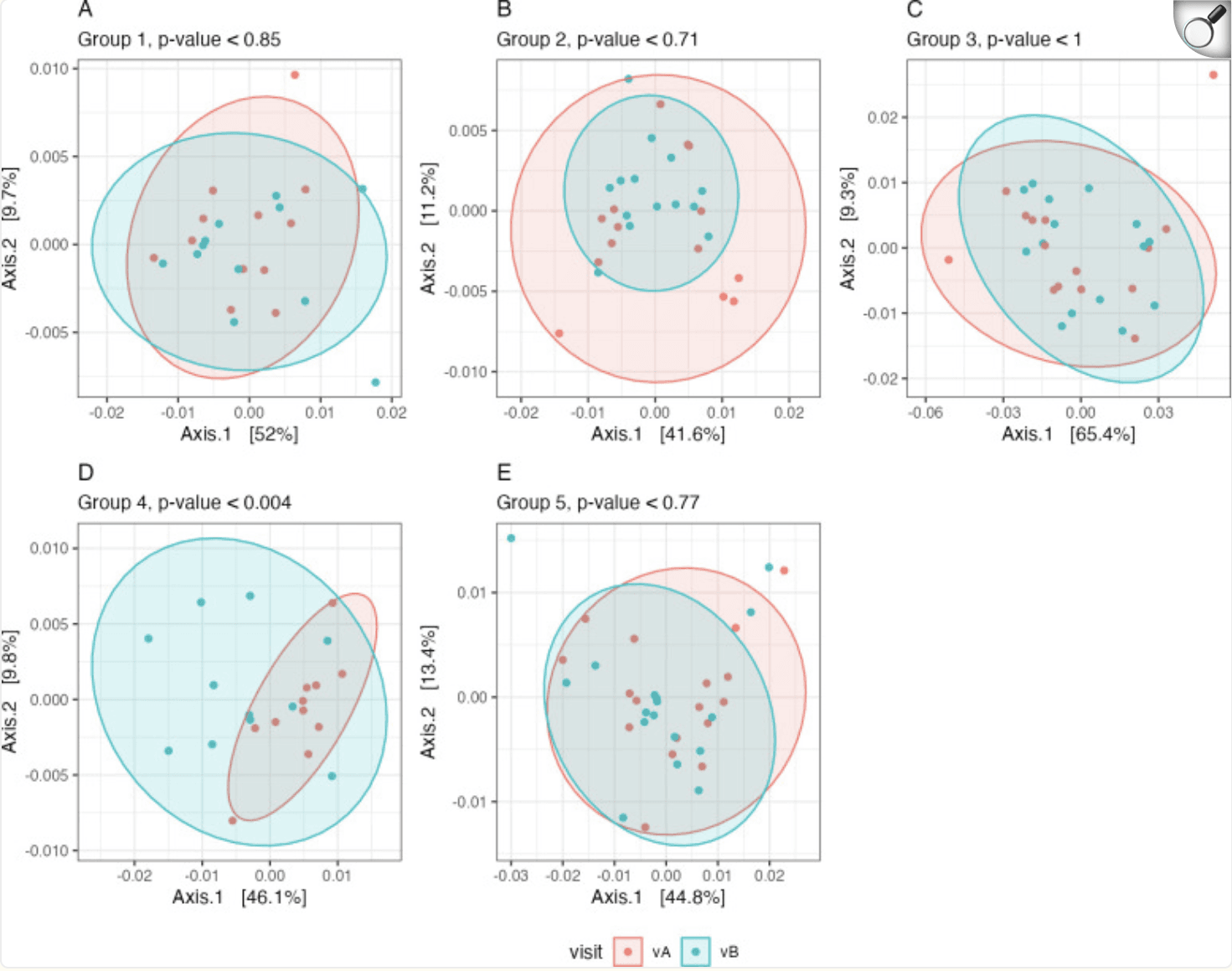
Beta diversity. The weighted UniFrac distance metric was used to estimate the dissimilarities between the samples of each group (A–E, groups 1–5) before (vA, red) and after (vB, blue) the intervention. Significance between visits was assessed with a PERMANOVA test |
From Gut to Game: Kefir’s Impact on Soccer Players’ Microbiome and VO₂max
In the quest to optimize athletic performance through nutritional strategies, a recent randomized controlled trial explored the effects of daily kefir consumption on gut microbiota and physical fitness in 21 professional female soccer players. Kefir, a fermented milk product rich in diverse probiotic strains, has been linked to improvements in lipid metabolism, inflammation, and gastrointestinal health. Until now, few studies have rigorously examined its impact on the microbiome–performance axis in elite athletes.
Participants aged 18–29 years were randomly assigned to consume 200 mL of kefir each day for 28 days (experimental group) or maintain their usual diet (control group). Before and after the intervention, researchers collected stool samples for 16S rRNA gene sequencing to profile the gut microbiome, conducted anthropometric measurements including body composition via bioelectrical impedance and skinfold calipers, and administered the 30-15 Intermittent Fitness Test to assess maximal aerobic speed (VO₂max) and finishing speed. Dietary intake was monitored through three-day food diaries to account for macronutrient influences.
The findings revealed that kefir consumption led to a notable increase in microbial diversity (Shannon and Chao1 indices) and a significant rise in beneficial taxa such as Akkermansia muciniphila and Faecalibacterium prausnitzii, both known for their roles in energy metabolism and anti-inflammatory pathways. Importantly, VO₂max and finishing speed correlated strongly with the abundance of short-chain fatty acid–producing bacteria, suggesting a link between microbiota modulation and enhanced athletic output. While body composition remained largely unchanged, the study underscores kefir’s potential as a functional food in sports nutrition. Further long-term investigations are warranted to confirm these benefits and elucidate underlying mechanisms.
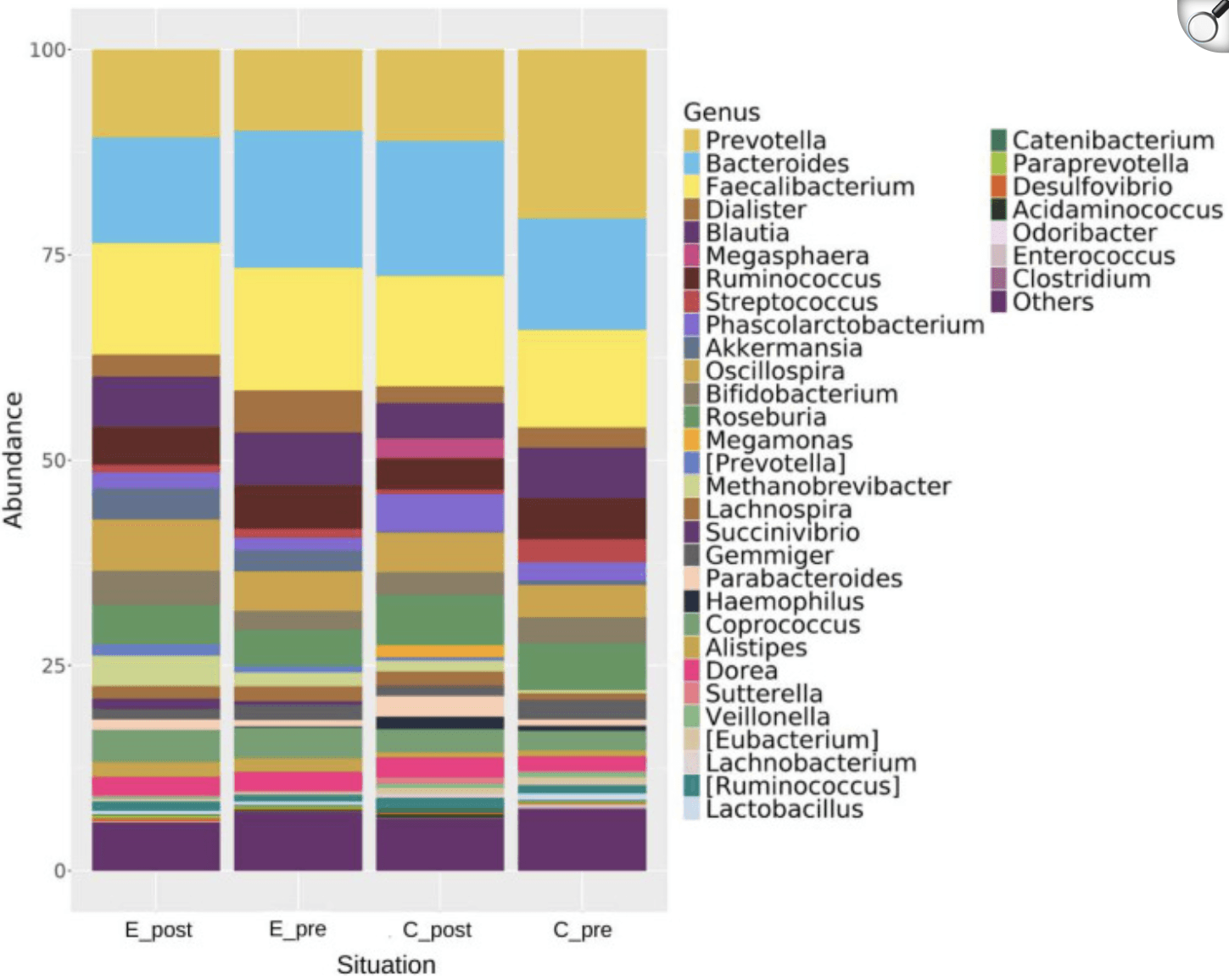
Relative abundance of bacterial genera across different conditions (E_post, post-experimental; E_pre, pre-experimental; C_post, post-control; C_pre, pre-control). Dominant genera, including Prevotella, Bacteroides, and Faecalibacterium, are highlighted alongside changes in other genera, including Akkermansia and Bifidobacterium. |
Rebalancing Young Guts: FMT Capsules Plus Partial Enteral Nutrition in Crohn’s
In paediatric Crohn’s disease (CD), disrupted gut microbial communities contribute to chronic inflammation, yet safe, non-invasive microbiome-targeted therapies remain scarce. In a 2025 single-centre, open-label trial, Zou et al. evaluated the feasibility and safety of oral faecal microbiota transplantation (FMT) capsules combined with partial enteral nutrition (PEN) in children aged 10–13 years with active CD. While standard care relies on immunosuppressants and steroids—with significant side-effect burdens—this study explored whether replenishing key microbial players could induce remission without invasive procedures,
Seventeen patients received six days of high-dose FMT capsules (3×10¹³ freeze-dried microbes per course) alongside PEN (covering 80% of caloric needs), and 16 controls received PEN plus conventional immunosuppression. Over 10 weeks, investigators tracked clinical (Paediatric CD Activity Index), endoscopic (Simple Endoscopic Score for CD), and biochemical markers (faecal calprotectin, CRP, ESR), along with immunological cytokines (IL-6, IL-10) and 16S rRNA–based gut microbiome profiling at baseline and post-treatment.
By week 10, clinical and endoscopic remission rates were comparable between groups, but the FMT arm exhibited significantly greater reductions in inflammatory markers (mean faecal calprotectin from ~2400 to ~475 µg/g; CRP and ESR likewise P < 0.05) and favourable cytokine shifts (↑ IL-10, ↓ IL-6). Importantly, α-diversity rebounded toward healthy benchmarks, and the relative abundance of six “core” SCFA-producing genera (Agathobacter, Akkermansia, Roseburia, Blautia, Subdoligranulum, Faecalibacterium) increased markedly, correlating with anti-inflammatory profiles. Mild constipation was the only common adverse event, underscoring the regimen’s tolerability. These findings position oral FMT capsules plus PEN as a promising, patient-friendly strategy to restore microbiota balance and attenuate inflammation in paediatric CD, warranting larger, multicentre trials to confirm long-term efficacy and mechanistic pathways.
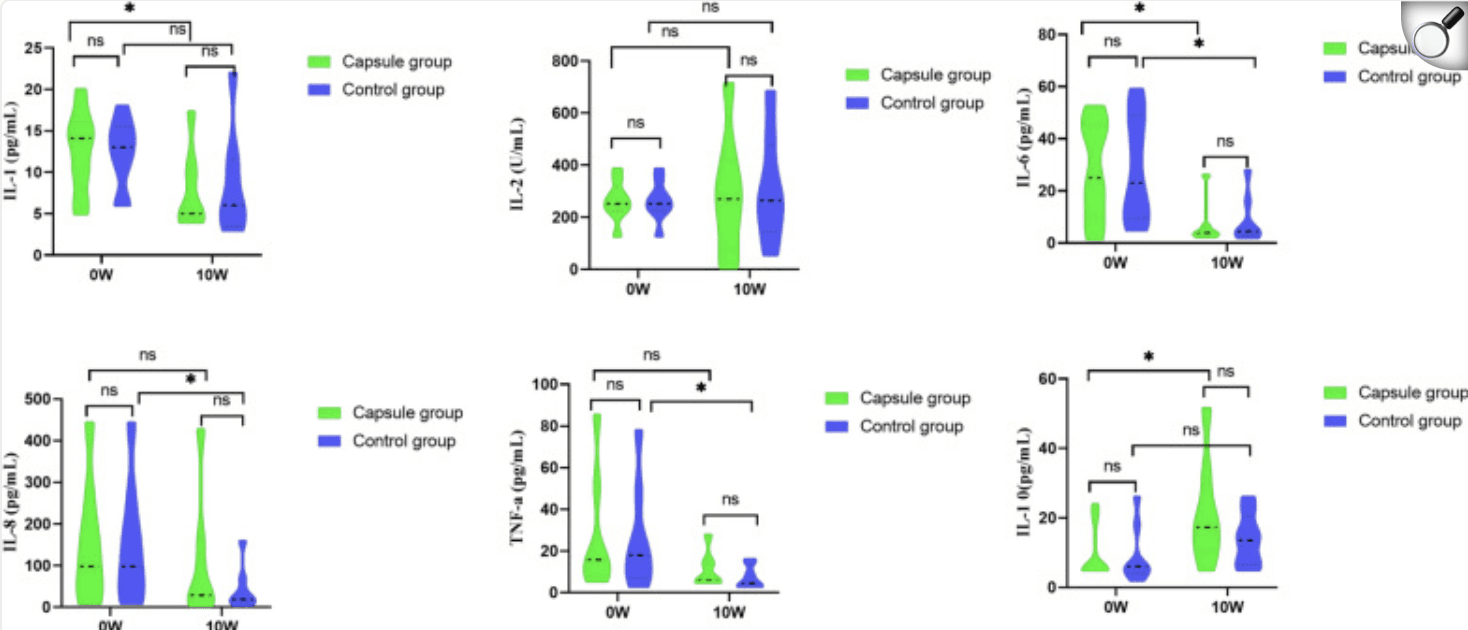
| Changes in the serum cytokine before and after treatment of the two groups. (The comparison between the two groups was the Mann-Whitney test, Paired Wilcoxon rank sum test was used before and after treatment, ***P < 0.001. |
Precision Nutrition: Using Your Microbiome to Decode Carb Sensitivity
Dietary composition and the gut microbiome are intricately linked to how our bodies regulate blood sugar, yet individual responses can vary widely. To address this variability, researchers conducted a series of nutritional “n-of-1” trials, where each participant serves as their own control. Thirty adults cycled through high-carbohydrate (HC) and low-carbohydrate (LC) diets in randomized, crossover periods spanning 72 days, allowing for precise, within-person comparisons of how diet shapes both microbial communities and glucose levels.
Over the intervention, participants wore continuous glucose monitors and provided stool samples for shotgun metagenomic sequencing. This dual approach captured real-time blood sugar fluctuations alongside detailed microbiome profiles. Analysis revealed that each individual’s gut microbiota responded uniquely to the HC and LC diets—some microbes bloomed with high carbs, others diminished. Leveraging these personalized shifts, the team calculated a “carb-sensitivity score” based on key bacterial signatures, which closely tracked post-meal glucose excursions during the HC phases but was unrelated under LC conditions.
To ensure broader relevance, the carb-sensitivity score was tested in an independent cohort of 1,219 people with existing metagenomic data. It robustly predicted glycaemic responses to carbohydrate intake, underscoring its potential as a biomarker for tailoring dietary advice. By demonstrating that high-carb diets modulate gut microbes in a person-specific manner—and that these microbial changes mirror blood sugar control—this work pioneers a precision nutrition framework. Future studies can build on this paradigm, integrating personalized microbiome insights to optimize diet plans for metabolic health.
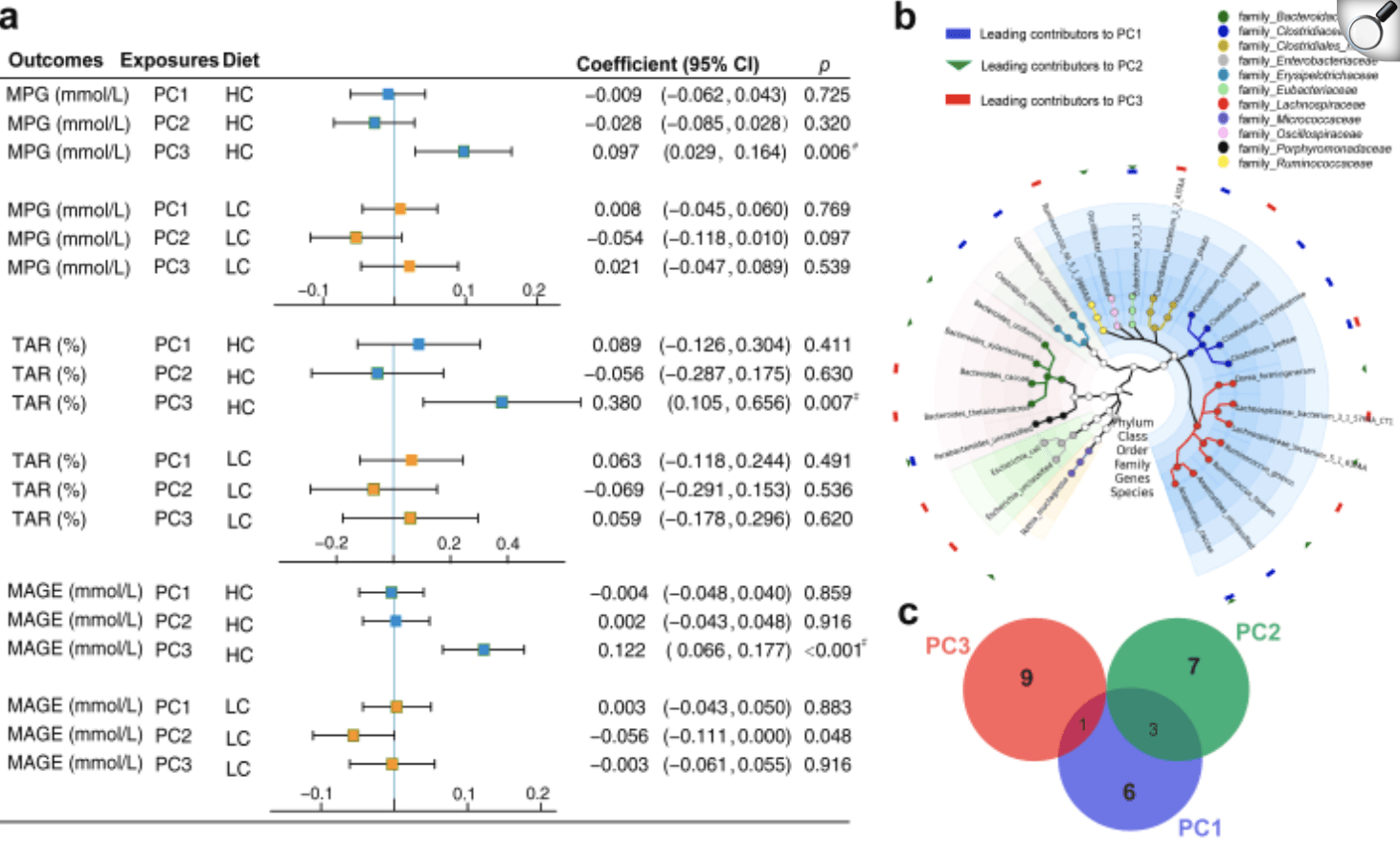
| Associations of the dimensionality-reduced gut microbial features with postprandial glycaemic responses and configurations of the features. (a) Forest plots presenting associations of the dimensionality-reduced gut microbial features (top 3 principal components) with the maximum postprandial glucose (MPG), the time above range (TAR) and the mean amplitude of glycaemic excursions (MAGE), stratified by the interventional diets (HC vs. LC). The linear mixed model was used to assess the associations of each top 3 PC (z-score standardized) with MPG, MAGE and TAR, respectively, adjusted for age, sex, BMI and physical activity. Error bars are beta coefficients with 95% confidence intervals. 28 participants with repeat measures were included in this analysis, and # indicates the Bonferroni corrected p value < 0.05; The adjusted p value was obtained by multiplying the raw p value by the number of independent tests (3 outcomes∗2 models). (b) Taxonomic tree based on the collection of 26 leading species that contributed substantially (top 10) to the PC1, PC2 or PC3. The nodes from the inner to the outer circles represented the bacterial taxa from the phylum to the species level, with the nodes at genus and species level coloured according to their assignment to families. The notes of bacterial families corresponding to the colour of nodes are located at the upper right corner of the graph. The blue rectangles, green triangles and red rectangles on the outermost ring denote the leading contributors to PC1, PC2 and PC3 respectively. (c) Venn plot indicating common and specific of leading contributors to PC1, PC2 and PC3. |
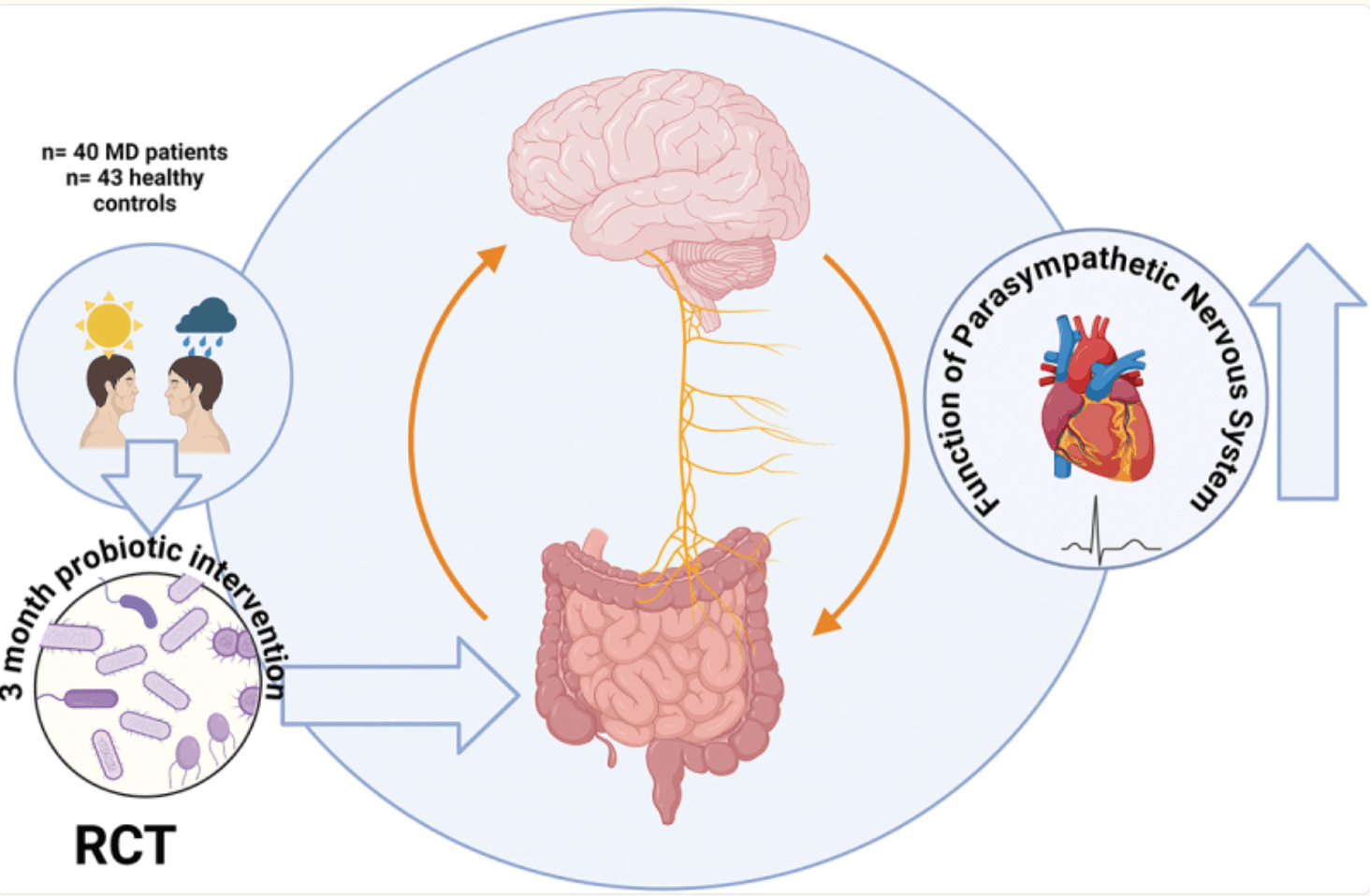
Stress Repair from Within: Boosting Vagal Tone with Multispecies Probiotics
In major depression (MD), disruptions in the gut–brain axis and autonomic regulation have been implicated in symptom severity, yet few interventions target these pathways directly. This randomized, placebo-controlled trial by Mörkl et al. investigated whether a multispecies probiotic supplement (OMNi-BiOTiC® STRESS Repair) could enhance vagus nerve (VN) function—a key conduit of gut–brain communication—in both MD patients and matched healthy controls (HC). Over three months, 86 adults (43 MD, 43 HC) received either probiotic or placebo capsules twice daily, with assessments at baseline, one week, 28 days, and 12 weeks.
Participants underwent 24-hour electrocardiography (ECG) to measure heart rate variability (HRV)—a proxy for VN activity—and provided stool samples for 16S rRNA sequencing to profile microbial shifts. Clinical and sleep parameters were also recorded, including the Pittsburgh Sleep Quality Inventory (PSQI). By combining continuous HRV monitoring with microbiome analyses, the study could link physiological VN changes to specific alterations in gut bacterial communities.
After 12 weeks, MD patients taking the probiotic exhibited significantly higher morning HRV indices—indicative of improved VN function—compared to both their baseline and placebo counterparts. Microbiome data revealed increased richness of the order Christensellales, notably Akkermansia muciniphila, which correlated with HRV gains. Moreover, probiotic-treated MD participants reported improvements in sleep latency and reduced reliance on sleep medications. Adverse events were minimal and balanced across groups. These findings suggest that targeted probiotic therapy may stimulate VN activity through favourable gut microbial modulation, offering a non-invasive adjunctive approach for managing depressive symptoms. Future larger-scale studies are warranted to confirm these mechanisms and explore longer-term clinical benefits.
Graphical Abstract
Gut Guard: Dietary Fibre's Role in Suppressing Oral-Origin Bacteria in the Colon
Diets rich in fibre, fruits, and vegetables have been linked to beneficial shifts in the gut microbiome, yet most intervention studies focus on faecal samples rather than the microbes residing in the colorectal tissue. The Polyp Prevention Trial—a 4-year, randomized clinical trial—addressed this gap by assigning participants with recently removed adenomatous polyps to a high-fibre (≥18 g/1000 kcal), high-fruit and vegetable (≥3.5 servings/1000 kcal), low-fat (≤20% of kcal) diet or to general dietary guidance. Rectal biopsies were collected at baseline, year 1, and year 4 from over 450 individuals to characterize tissue-associated bacteria via 16S rRNA gene sequencing .
Using repeated-measures linear mixed-effects models and PERMANOVA tests, the investigators evaluated changes in alpha diversity (species richness and evenness), beta diversity (community composition), and the relative abundance of pre-specified genera known to originate in the oral cavity or be implicated in colorectal cancer—such as Porphyromonas, Prevotella, and Fusobacterium. Analyses were adjusted for within-participant correlations and explored both the full intervention arm and subgroups of adherent “super-compliers” .
Overall, the dietary intervention did not significantly alter tissue-level diversity metrics. However, it was associated with a stronger decrease in the relative abundance of two oral-originating genera previously linked to colorectal cancer: Porphyromonas (intervention effect at year 1 vs baseline: –0.24 ± 0.07; P = .004) and Prevotella (–0.40 ± 0.14; P = .01). These findings suggest that while broad community diversity in rectal tissue may be resilient to dietary change, specific potentially pathogenic taxa can be modulated by a high-fibre, high-plant, low-fat diet—offering a potential avenue for colorectal cancer prevention research.
