Holobiome: Research I Articles I Updates I News I Series I Feed
The Holobiome blog series offers an AI-assisted summary of the latest research articles on human microbiome.
Dual Therapy in Atopic Dermatitis: A Microbiome-Targeted Approach to Healing
A recent clinical study explored whether combining a topical antimicrobial with corticosteroid treatment could better support skin recovery in people with mild-to-moderate atopic dermatitis (AD). In this trial, 40 participants with mild-to-moderate AD were split into two groups—one treated with corticosteroids alone, and the other with both corticosteroids and a topical antimicrobial. Researchers collected skin swabs from both lesioned and non-lesioned areas before treatment, immediately after treatment, and two weeks later. These samples were analysed to track changes in bacterial and fungal communities using DNA sequencing.
While both treatment groups showed noticeable improvements in eczema symptoms and skin barrier function, there was a striking difference in how the skin microbiome responded. The group receiving combination therapy showed a significant restoration of microbial balance, including an increase in beneficial sebum levels and a decrease in harmful bacteria like Staphylococcus. The steroid-only group, although clinically improved, did not show the same correction in microbial imbalance. This suggests that inflammation alone may not be the only target in AD-supporting a healthy skin microbiome might be equally important for long-term recovery.
The study confirms that AD lesions tend to have reduced sebum, lower levels of Cutibacterium, and elevated Staphylococcus, all signs of microbial disruption. By addressing both inflammation and microbial dysbiosis, the combination treatment appears to offer a more holistic path to skin healing. These findings open the door to future eczema treatments that go beyond symptom control and aim to restore the skin's natural ecosystem. More research is needed, but this dual-approach may become an important step forward in managing AD.
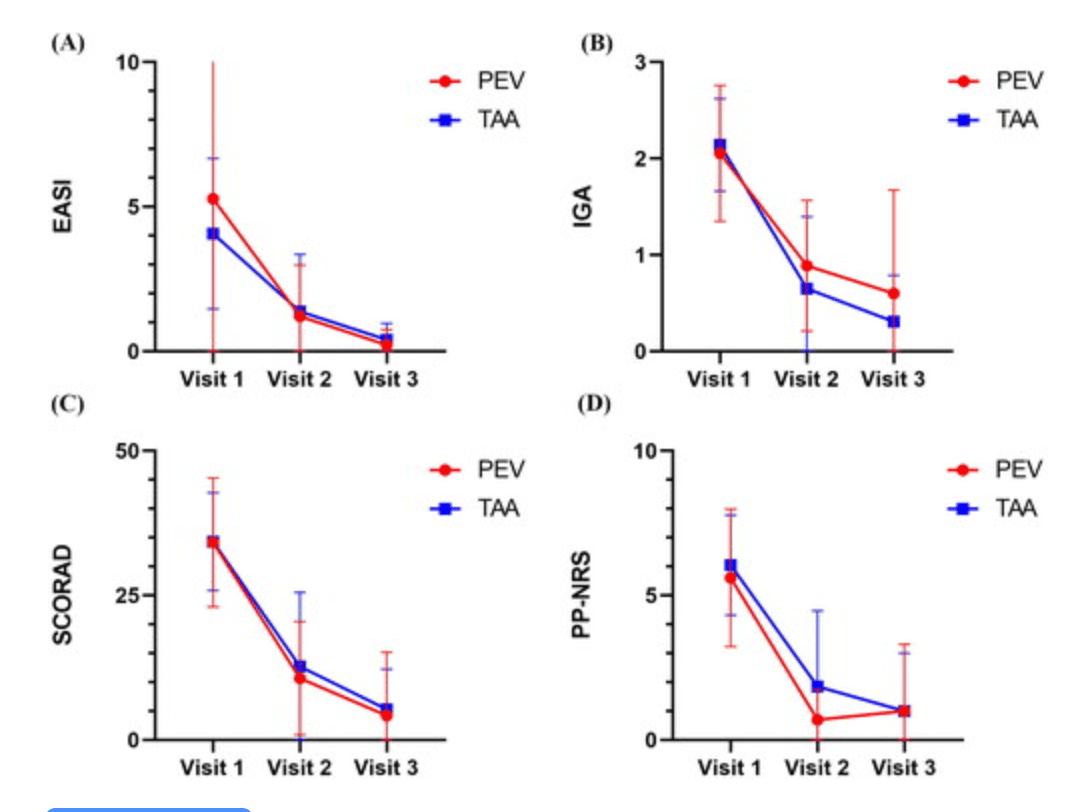
Figure 1. Compare the disease severity (EASI, IGA, SCORAD) and itch score (PP-NRS) of AD patients after using topical Pevisone and topical triamcinolone acetonide cream. PEV: Pevisone ; TAA: triamcinolone acetonide acetate. |
Integrating Probiotics into Acne Care: Evidence from a Double-Blind Clinical Trial
In a randomized, double‑blind clinical trial, 80 individuals with moderate acne were split into two groups to test whether adding a probiotic to standard treatment could improve outcomes. All participants used a daily antibacterial face wash and alternate-night adapalene gel. One group took doxycycline (100 mg) daily, while the other combined doxycycline with a daily probiotic capsule. Acne severity was assessed at the start and after 1, 2, and 3 months using standardized measures like the Global Acne Grading System (GAGS) and a morphological acne grading method.
Both groups saw overall improvement in acne scores, but the probiotic-augmented group showed significantly greater reductions—especially on the forehead (p = 0.018), chin (p = 0.021), and nose (p = 0.021). Severity of lesions dropped more sharply in this group (p < 0.001), whereas changes on the cheeks, back, and chest were similar between groups. Importantly, adding probiotics didn’t lead to any side effects
These findings suggest that probiotics can safely enhance the effectiveness of routine acne care-likely through modulation of the skin and gut microbiome-without increasing adverse events. The probiotic boost appears particularly beneficial for facial acne, making it a promising addition to treatment regimens. While further large-scale studies would help validate and refine these results, this trial highlights a potential shift toward integrative approaches that combine traditional therapies with microbiome-targeted strategies for improved acne management.
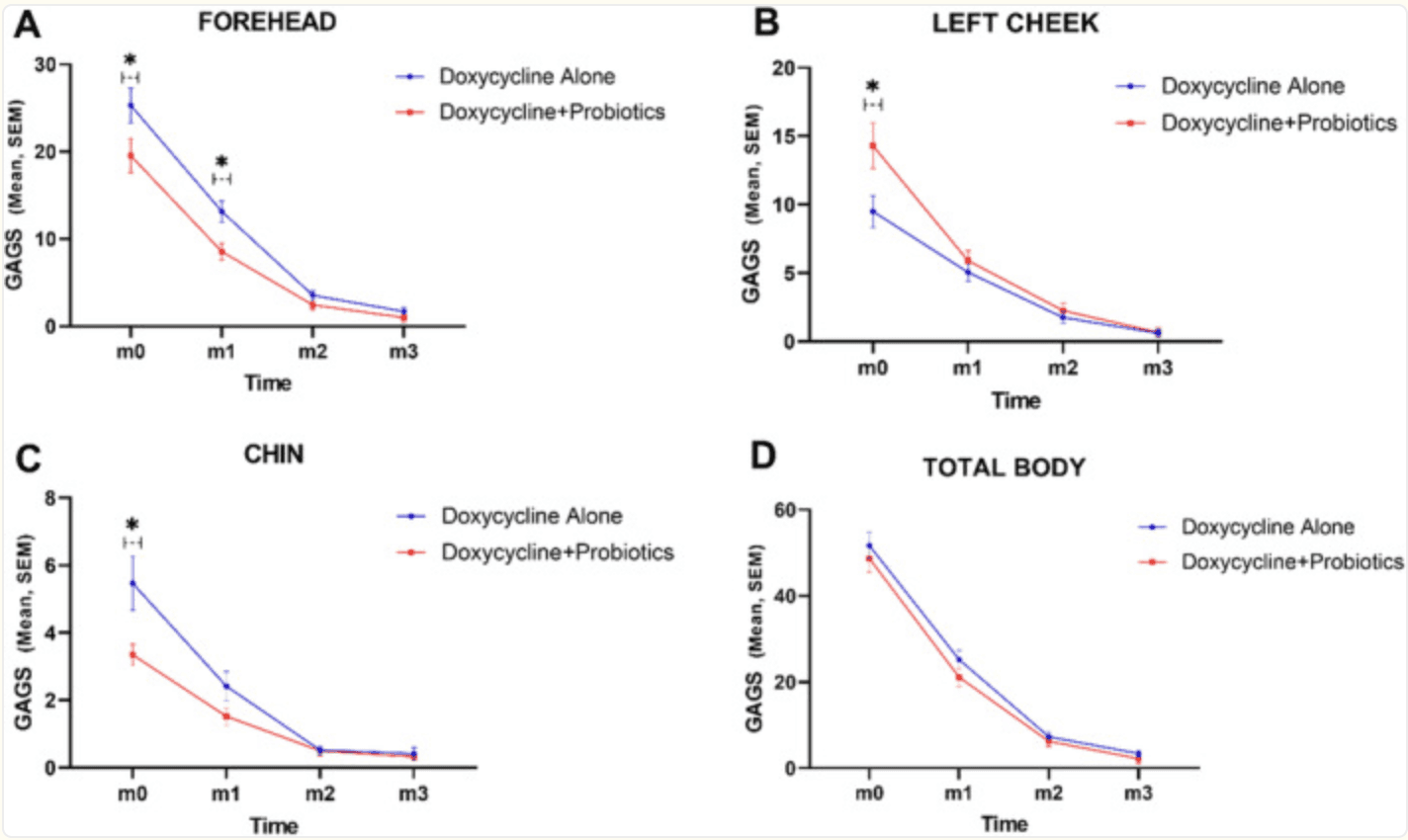
Charts represent mean and standard error of mean (SEM) GAGS score in forehead (A), left cheek (B), chin (C), and total body (D); *p‐value < 0.05. |
Gut Microbiome Modulation by Micronutrients in ADHD: A Sub-Analysis of the MADDY Trial
A recent sub-analysis of the Micronutrients for ADHD in Youth (MADDY) randomized, double‑blind trial explored how broad-spectrum micronutrient supplementation affects the gut microbiome in children (ages 6–12) with ADHD and emotional dysregulation . Forty-four participants (33 on micronutrients, 11 on placebo) provided stool samples at baseline, 8 weeks, and – for the placebo group – 16 weeks. Using 16S rRNA V4 sequencing, the researchers tracked changes in microbial diversity (alpha and beta diversity) and specific bacterial taxa. They found that micronutrient supplementation significantly increased microbial evenness (alpha diversity) and shifted overall community structure (beta diversity) compared to placebo.
Drilling deeper, the study revealed a notable decrease in the phylum Actinobacteriota in the micronutrient group compared to placebo. Additionally, two families of butyrate-producing bacteria-Rikenellaceae and Oscillospiraceae-showed a significant increase specifically in kids who responded behaviourally to micronutrients versus those who did not. These changes hint at a microbial mechanism underpinning symptom improvement: boosting butyrate producers (known for gut‑brain benefits) while reducing potentially overrepresented phyla in ADHD .
In summary, this is the largest study to date examining microbiome shifts in paediatric ADHD following micronutrient supplementation. It shows that such supplements do more than support basic nutrition—they can reshape gut ecosystems in ways linked to clinical improvement. While these findings are promising, further research will be needed to confirm causality, replicate results, and explore whether targeting the microbiome could become a mainstream complement to ADHD care.
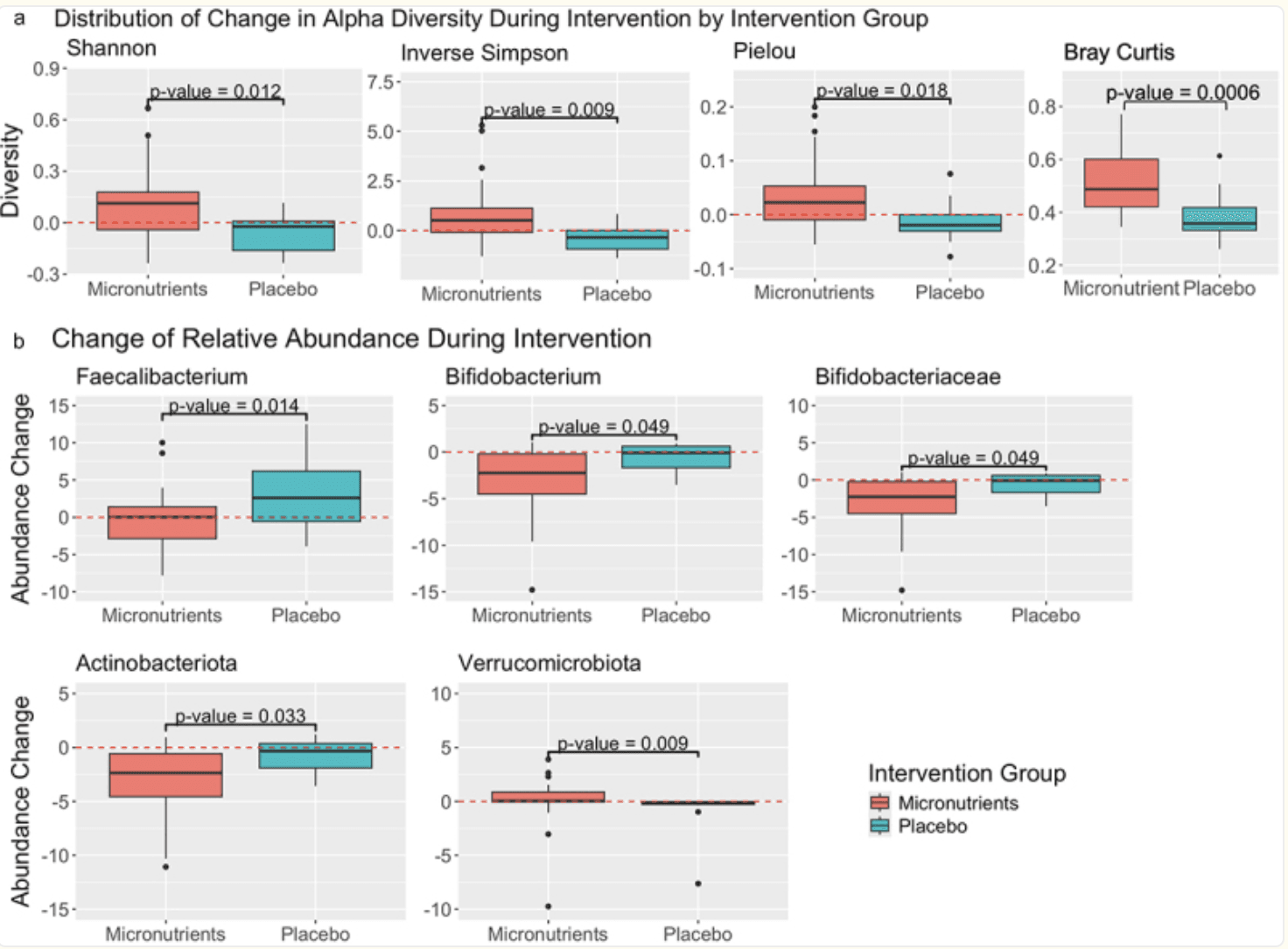
| Distribution of change in alpha diversity and abundance of specific taxa baseline to week 8 by micronutrients and placebo. A) the distribution of change in alpha diversity (within sample community diversity) during intervention for each intervention group at the family level. See table S3 for median [IQR] values. The Y-axes represent the change in each respective alpha diversity metric during intervention (value after intervention – value before intervention = change during intervention). B) the distribution of change in relative abundance during intervention for each intervention group, for select taxa that exhibited a significant difference between intervention groups. Faecalibacterium and Bifidobacterium are genera, Bifidobacteraceae is a family, and Actinobacteriota and Verrucomicrobiota are phyla. The Y-axes represents the change in relative abundance during intervention (relative abundance after intervention – relative abundance before intervention = change in relative abundance during intervention). In both figures, the X-axis represents the response group for which each distribution belongs to. Those subjects in the “placebo” intervention group received a placebo during the timeframe of this analysis, whereas subjects in the “micronutrients” intervention group received micronutrients. Zero (indicating no change) is indicated by a red dashed line. P-values here were not corrected for multiple testing and correspond to the significance of the difference in distribution of change in alpha diversity, between intervention groups, as calculated using a Wilcoxon rank-sum test for significance. |
Postpartum Stress Alters Breast Milk Microbiome: Implications for Infant Gut Colonization
A recent prospective observational study explored how maternal stress during the early postpartum period might influence the microbiome of human breast milk—a crucial factor in shaping infant gut colonization. Researchers recruited 92 lactating mothers, dividing them into high-stress (n=23) and control (n=69) groups. The high-stress group included mothers whose infants had been hospitalized for at least two days. Stress levels were assessed using validated questionnaires and cortisol measurements (hair, saliva, and milk), while breast milk samples were collected on postpartum days 10 and 24. Using 16S rRNA sequencing, the team analysed how milk microbiome diversity and composition varied between the two groups.
The findings revealed significant differences in microbial community structure: milk from stressed mothers showed lower levels of Streptococcus, Gemella, and Veillonella, and higher levels of Staphylococcus, Corynebacterium, and Acinetobacter. Beta-diversity analyses confirmed that stress was the strongest correlate of microbiome variation, though maternal education level and infant sex also played roles. Interestingly, milk cortisol levels did not correlate with microbiome composition, suggesting that maternal perception or environmental stress might influence milk microbes in ways not directly tied to milk cortisol. These alterations in milk bacterial composition may impact how infants’ guts are seeded early in life.
In short, this study provides preliminary evidence that early postpartum stress alters breast milk’s microbial makeup in ways that could influence infant development and long-term health. It highlights the potential importance of supporting maternal mental well-being—not just for the mother's sake, but also for the microbiome-dependent aspects of infant health. Further research is needed to clarify whether these microbiome changes translate into measurable outcomes in infant growth, immunity, or neurodevelopment.
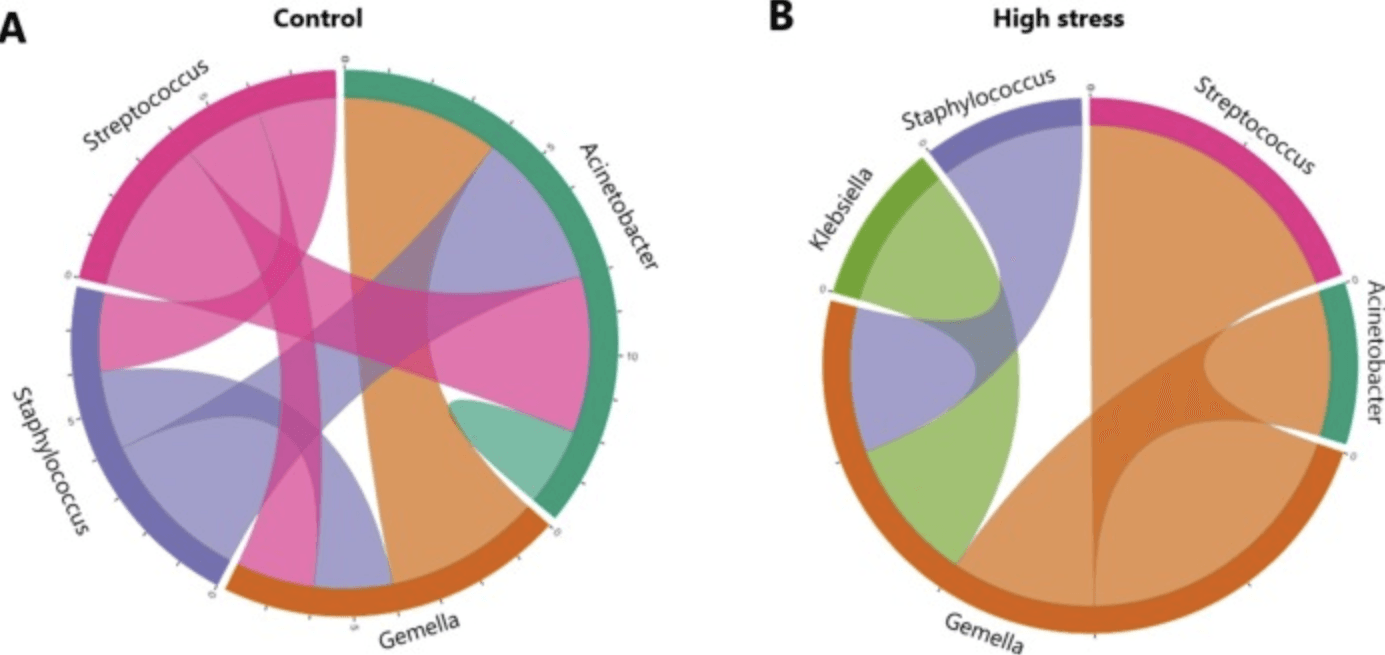
Fig. 4. Inter-bacterial interactions. Bacterial abundance correlation networks from control (A) and high stress (B) samples summarized at genus-level. Summary of the number of detected edges between ASVs in BEEM-Static interaction network analysis, coloured by genus. |
Blueberry-Induced Microbial Changes in Older Adults: A Pilot Study on Metabolic Outcomes
In a 12-week randomized, double‑blind, placebo‑controlled study involving 55 sedentary adults aged over 60 with overweight or obesity, participants consumed either daily blueberry powder (equivalent to 1.5 cups of fresh blueberries) or an indistinguishable placebo, alongside supervised exercise. While overall gut microbiome composition and diversity remained unchanged, those in the blueberry group experienced a significant increase in Coriobacteriales incertae sedis (p = 0.049)—a bacterial group involved in polyphenol metabolism. This suggests that blueberry polyphenols may enhance the gut's capacity to process these beneficial compounds.
Regarding cardiometabolic markers, beta‑hydroxybutyrate, TMAO, and related metabolites showed no significant shifts in the blueberry group . Interestingly, the placebo arm saw reductions in total cholesterol, LDL, non‑HDL, LDL particles, and ApoB, whereas the blueberry arm noted decreases in total HDL particles and ApoA‑I.
Furthermore, within the blueberry group, increased Coriobacteriales levels correlated with fewer large LDL particles post‑prandially, although no consistent cardiovascular benefits were detected. The authors conclude that while blueberry intake may boost gut microbiome function related to polyphenol metabolism, larger and longer trials are essential to determine real-world impacts on cardiometabolic health.
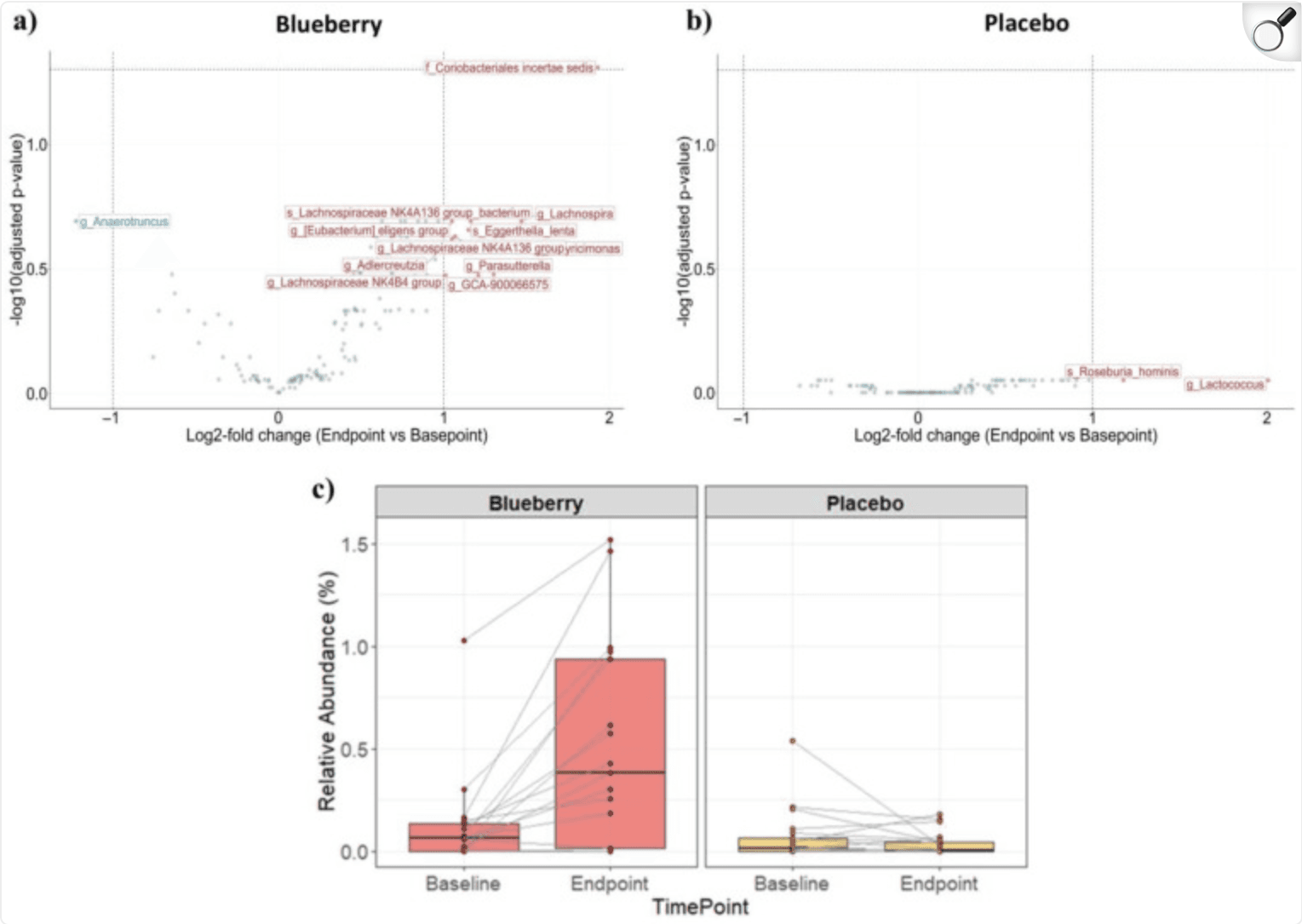
Differential abundance analysis of microbes. Volcano plots with log2 fold change (L2FC) (endpoint vs. baseline) on the x-axis and −log10-adjusted p-value (Benjamini–Hochberg correction) on the y-axis for two treatment groups; (a) blueberry group (left panel) and (b) placebo group (right panel). Data points represent taxa, which were aggregated using the lowest taxonomic rank classified for each amplicon sequence variants and are labeled with prefixes indicating the rank (‘s’ = species, ‘g’ = genus, ‘f’ = family). Colors indicate whether taxa are enriched (red, L2FC ≥ 1) or depleted (blue, L2FC ≤ −1) in endpoint samples relative to baseline samples. Light green taxa are those that did not have a large effect size (−1 < L2FC < 1). (c) Relative abundance of Coriobacteriales incertae sedis between baseline and endpoint samples for the blueberry group (left panel) and placebo group (right panel). Points represent individual samples. Lines connect samples from the same subject. |
