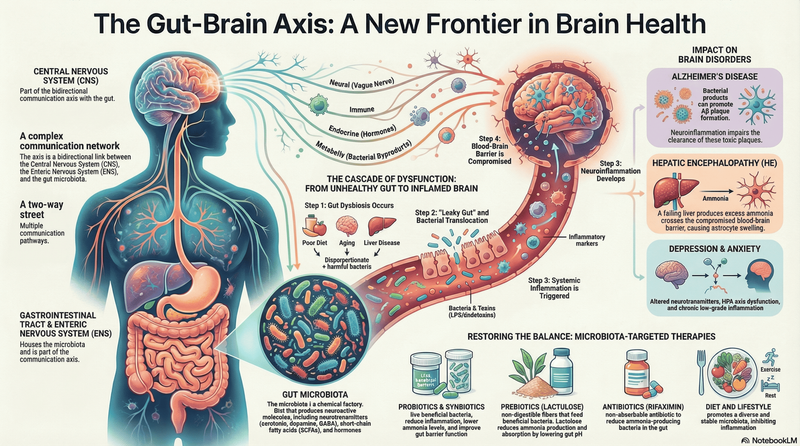
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Can Your Gut Bacteria Improve Your Metabolism? Chicory Says Yes!
When people at risk of type 2 diabetes consumed a simple supplement made from dried chicory roots which contain a lot of so-called “intrinsic fibers” (the fiber enclosed inside intact plant-cell walls, not isolated fiber powders) their gut microbes responded in a distinct and healthy way. Over 12 weeks, the amounts of friendly bacteria like Bifidobacterium species and Anaerostipes species rose dramatically. By week 12, Bifidobacterium levels had increased roughly four-fold, and Anaerostipes had risen about 3–4 times compared with baseline.
This shift was not just cosmetic, it changed how the gut processed fiber. The microbial community developed greater capacity for producing beneficial short-chain fatty acids (SCFAs), especially Butyrate. Fecal butyrate increased by around 17 % after 12 weeks of fiber intake, along with rises in other SCFAs such as acetate and propionate. Butyrate and other SCFAs are important because they feed and support the cells lining the colon and help maintain gut health.
These microbiome changes coincided with small but hopeful signs of better metabolic health: people showed improved whole-body insulin sensitivity, modest reductions in blood triglycerides, and a trend toward lower liver fat. The idea is that by tweaking the gut ecosystem boosting “good” fiber-eating, butyrate-producing microbes the body’s response to insulin and overall metabolism may improve.
In everyday terms, the study suggests that eating fiber-rich foods with their natural structure not overly processed, but more like whole-food roots could feed and support helpful gut microbes that make butyrate, potentially nudging your metabolism into better shape. It adds to the growing idea that gut health and metabolic health go hand in hand.

Graphical Abstract
Why Giving Your Gut a Break May Help Your Brain Think Better
When people with obesity adopted an alternate-day fasting (ADF) routine, their gut microbe community changed noticeably and these changes seemed to go hand in hand with clearer thinking and sharper mental function. Over time, certain “friendly” bacteria increased, while those more often associated with inflammation or metabolic imbalance decreased. That shift resulted in a more balanced, diverse microbial ecosystem something scientists interpret as a sign of a healthier gut.
The richer, more balanced microbiome didn’t just sit there quietly it appeared to actively support improved brain health. In experiments on mice, the beneficial effects of fasting on memory and cognition disappeared when the gut microbes were wiped out, showing that the microbes were the key bridge between diet and brain. The microbes seemed to influence the brain’s immune cells (microglia), helping them behave in ways linked to reduced inflammation and better brain cell maintenance.
In simpler terms: by gently reshuffling who lives in our gut inviting in helpful microbes and pushing out the “troublemakers” fasting may help tune our inner ecosystem in a way that benefits not just digestion or metabolism, but even our brain. For anyone curious about gut health and mindful eating or fasting, the study offers a compelling hint: the gut microbiome might just play a powerful role in how our brains stay healthy.
How Postbiotics Help Your Gut Microbes Get Moving
In people (and in mice) with constipation problems, taking the postbiotic changed which kinds of microbes lived in the gut. Specifically, the supplement helped boost bacteria often considered helpful including members of the genus Bifidobacterium while reducing bacteria that tend to be over-represented when digestion is sluggish. This shift made the community of gut bacteria more balanced, which is generally a sign of a healthier microbiome.
Because the gut microbes changed, the gut environment itself improved: digestion became smoother, and the severe constipation symptoms many suffered seemed to ease. In the mouse experiments, these microbial shifts were tied directly to better gut movement and stool consistency suggesting that the bacteria played a real, active role in improving bowel function, not just a side effect.
In plain terms: the study shows that nudging your gut bacterial community with the right kind of supplement, one that doesn’t rely on live bacteria, but on substances produced by bacteria (“postbiotic”) can help restore balance in the gut flora. That balance, in turn, can support healthier digestion and relieve persistent constipation. It’s a nice reminder that our gut microbes are not just passive hitchhikers, they can be guided to work more constructively for us.

Graphical Abstract
Beyond Probiotics: The New Way Researchers Are Transforming the Gut Microbiome
Our guts are home to a teeming universe of microbes and what we eat influences which ones thrive. In the recent study published as “Effects of synbiotics surpass probiotics alone…” researchers explored how combining prebiotics and probiotics (aka “synbiotics”) changed the gut microbiome more than probiotics alone. Their main finding: when people took synbiotics, their gut microbial community shifted showing both different species present and a change in overall diversity.
Under the synbiotic treatment, beneficial microbes increased likely species that are known to help digestion and overall gut health. Meanwhile, the overall mix of microbes became more balanced, reducing the overgrowth of potentially harmful microbes. This kind of rebalancing more “friendly” bacteria, less “risky” ones tends to be associated with positive metabolic and immune effects.
Why does this matter? A healthier, more balanced gut microbiome can have ripple effects across overall wellbeing. With a favorable microbial mix, your gut may digest food better, produce beneficial metabolites, and keep inflammation in check. That in turn can support better energy use, gut comfort, and even help maintain a healthy immune system. Especially in a world where diet, stress, and antibiotics can disturb that community, such interventions might help steer your microbiome back to a healthy state.

Effect of MN-Gup and MN-Gup-GOS intervention on gut microbiome in fecal samples. Pre-intervention (a) and Post-intervention (b) PcoA analysis at the OUT level. PcoA analysis was performed based on the Bray–Curtis distance algorithm, and Nonparametric Multivariate Analysis of Variance was used to analyze the significant difference between groups. Changes in the abundance of the phylum Actinobacteriota (c), the genus Bifidobacterium (d) and Dorea (e) before and after the intervention in the placebo, MN-Gup, and MN-Gup-GOS groups. Comparison between groups over the same period of the characteristic genus bacteria was carried out by the Kruskal–Wallis H Test of non-parametric ANOVA with the Tukey–Kramer Post-hoc test. Mann–Whitney U test was used to compare the groups before and after the intervention. f Linear discriminant analysis effect size (LefSe) Reveals a Differential Abundance of Bacterial Taxa in Placebo, MN-Gup, and MN-Gup-GOS Groups Before and After Intervention. The significance level was set at Linear discriminant analysis (LDA) ≥ 2.0 for this LefSe analysis. g Heat map depicting correlation among glucose metabolism, bile acids, and gut microbiota. The correlation coefficient matrix was calculated using Pearson's method. The significance test of correlation coefficients is denoted as follows: ∗ for P < 0.05, ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001.
Stay tuned to unravel the latest discoveries on dynamic human-microbe interactions!