The MicroByte Series: From Dairy Starter to Probiotic Powerhouse: Lactococcus lactis
History
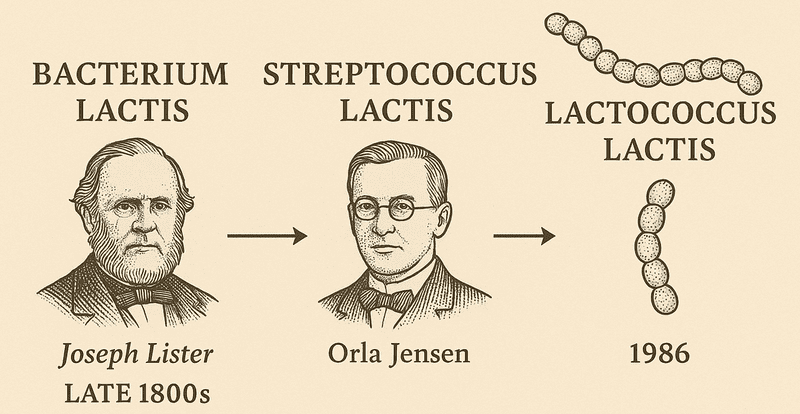
The story of the humble dairy starter, now known as Lactococcus lactis, is a thrilling snapshot of microbial science history. Its scientific journey began in the late 1800s when Joseph Lister, the father of antiseptic surgery, first identified the organism involved in fermentation, initially calling it Bacterium lactis. For a long time, however, it lived under the name Streptococcus lactis—a classification given by the Danish microbiologist Orla Jensen, who grouped it with other lactic acid-producing cocci based on its simple, bead-like shape. The formal shift to the currently accepted nomenclature occurred in 1986, when Schleifer and colleagues established the distinct genus Lactococcus. This molecular reclassification was based on deep phylogenetic analysis, separating these organisms from true Streptococcus species while retaining them within the order Lactobacillales. The progression in nomenclature—from Bacterium to Streptococcus to Lactococcus—is a direct chronicle of the advancement in microbial science, moving from basic morphological observation to comparative genomics. The current status provides a more accurate reflection of the organism’s distinct genetic and metabolic profile relative to other members of the Streptococcaceae family.
Health Benefits
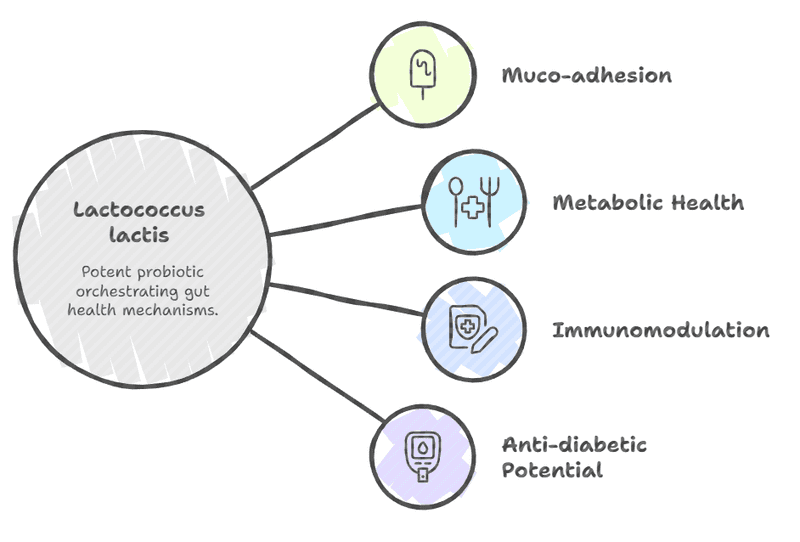
Lactococcus lactis acts as a potent probiotic by orchestrating a sequence of highly specific and synergistic mechanisms that bolster gut health, commencing with muco-adhesion, the critical first step involving the temporary colonization of the intestinal epithelium; this is achieved through cell-wall anchored proteins, such as the aggregation factor, which increases cell surface hydrophobicity to facilitate non-specific interactions with the mucus layer, ensuring the localized delivery of beneficial metabolites. A striking example of the organism's genomic economy is the cell-wall proteinase (PrtP), an enzyme vital for casein degradation in industrial settings, which simultaneously modifies the cell's physico-chemical properties, resulting in greater hydrophobicity and demonstrably increasing adherence to biotic surfaces like mucin. Once established, L. lactis, like other Lactic Acid Bacteria (LAB), contributes significantly to the gut's metabolic health by promoting the bioproduction of Short-Chain Fatty Acids (SCFAs)—key metabolites (e.g., butyrate) that nourish colonocytes, maintain intestinal barrier function, and provide valuable antioxidant activity while lowering the genotoxicity of fecal water. Furthermore, specific strains, such as L. lactis subsp. lactis, display sophisticated immunomodulation capabilities, showing promise in stimulating proinflammatory cytokine production and modulating overall immune and inflammatory signaling pathways, suggesting potential as a novel therapeutic agent for immune-related disorders; however, this promise is tempered by the vital caveat of high strain-specificity, requiring rigorous clinical validation for each proposed application, as highlighted by instances where one formulation (L. lactis spp. cremoris) failed to show significant effects on T-regulatory cells in healthy human subjects, underscoring the necessity of strain-level testing. A 6-week study on mice revealed the powerful anti-diabetic potential of a 14-strain probiotic isolated from fermented camel milk. This diverse microbial mix, featuring key gut-health champions like Lactococcus lactis, significantly improved both blood glucose and blood lipid parameters. This finding suggests that these traditional camel milk microbes could serve as a promising natural intervention to delay the development and progression of Type 2 Diabetes (T2D).
Industrial Application
Lactococcus lactis is indispensable to the dairy industry, serving as the primary organism in mesophilic starter cultures for fermentation at moderate temperatures. Its fundamental role is the rapid conversion of lactose into lactic acid, a process that is critical for two reasons: preservation, as the resulting low inhibits spoilage and pathogenic organisms; and coagulation, as the acid destabilizes casein micelles to form the curds essential for the structure of cheeses like Cheddar and Gouda. Beyond simple acidification, specific biovars are selected for complex flavor development. Notably, L. lactis subsp. lactis biovar diacetyl lactis acts as a crucial co-starter because it possesses the extra ability to ferment citric acid into characteristic aroma compounds, most importantly diacetyl, which imparts the sought-after creamy or buttery flavor to products like sour cream. This citrate pathway also generates carbon dioxide, a gas vital for defining the texture of certain semi-hard cheeses (creating 'eyes') and for giving body to buttermilk.
Beyond its dairy roles, specific strains of Lactococcus lactis produce nisin, a crucial bacteriocin used for food bio preservation. This potent antimicrobial peptide effectively targets Gram-positive pathogens by binding to Lipid II (blocking cell wall formation) and simultaneously disrupting the bacterial cell membrane, leading to rapid cell death. This unique and powerful mechanism makes nisin invaluable for controlling tough foodborne threats, including spore-forming bacteria, securing its role as a cornerstone of food safety.
Microbe Profile
Shape: Spherical Shape
Gram nature: Gram Positive
Spore formation: No spore formation
Oxygen requirement : Anaerobic
Optimal temperature: 30 °C
Optimal pH: 6.3-6.9
Taxonomic Classification
Domain: Bacteria
Phylum: Bacillota
Class: Bacilli
Order: Lactobacillales
Family:Streptococcaceae
Genus:Lactococcus
Species:Lactococcus lactis
-Varsha V
Reference
Mahony, J., Bottacini, F., & van Sinderen, D. (2023). Towards the diversification of lactococcal starter and non-starter species in mesophilic dairy culture systems. Microbial biotechnology, 16(9), 1745–1754.
Samaržija, D., Antunac, N., & Havranek, J. L. (2001). Taxonomy, physiology and growth of Lactococcus lactis: a review. Mljekarstvo, 51(1), 35-48.
Mercier-Bonin, M., & Chapot-Chartier, M. P. (2017). Surface proteins of Lactococcus lactis: bacterial resources for muco-adhesion in the gastrointestinal tract. Frontiers in microbiology, 8, 2247.
Markowiak-Kopeć, P., & Śliżewska, K. (2020). The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients, 12(4), 1107.
Jeong, H., Kim, S., Hwang, U. S., Choi, H., & Park, Y. S. (2023). Immunostimulatory Activity of Lactococcus lactis subsp. lactis CAB701 Isolated from Jeju Cabbage. Microorganisms, 11(7), 1718
Kondrotiene, K., Zavistanaviciute, P., Aksomaitiene, J., Novoslavskij, A., & Malakauskas, M. (2024). Lactococcus lactis in Dairy Fermentation—Health-Promoting and Probiotic Properties. Fermentation, 10(1), 16.
Hassan, H., St-Gelais, D., Gomaa, A., & Fliss, I. (2021). Impact of Nisin and Nisin-Producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and Bacterial Ecosystem of Cheese Matrices. Foods (Basel, Switzerland), 10(4), 898