Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
When Diabetes Drugs Meet the Microbiome: Metformin’s Hidden Gut Effect
In this study of the diabetes medications Metformin and Liraglutide, researchers explore not just blood-sugar outcomes but the surprising shifts in the gut microbial community that accompany treatment. They found that metformin—which is often viewed purely as a metabolic drug—also alters the gut environment: it increases the relative abundance of certain bacterial genera previously linked with improved metabolic health, while reducing those tied to inflammation and worse glycaemic control. Though the paper doesn’t deeply identify every species by name, it emphasises the broader view that the gut microbiota are actively reshaped by treatment and may mediate how the host responds.
As the gut flora shifts, so do microbial-driven mechanisms: there is evidence that metformin enhances short-chain fatty acid (SCFA) production or at least modulates pathways that involve SCFAs, which are known to influence the host’s energy harvest, gut barrier integrity, and systemic inflammation. The authors suggest that these microbiome changes might help explain why some patients respond better than others, and why metabolites and microbiota composition deserve attention alongside classic glucose-insulin metrics. The gut epithelium and immune cross-talk with microbes may thus become a secondary yet important target of therapy.
What makes this paper compelling from a microbiome perspective is how it shifts the narrative: medications aren’t just acting on human cells, but also on the microbial ecosystem inside us—and that ecosystem in turn influences drug efficacy and host physiology. For anyone curious about the gut microbiome’s role in chronic disease, it paints a clear message: we need to think of treatments as ecosystem-modifiers, not just chemical interventions.
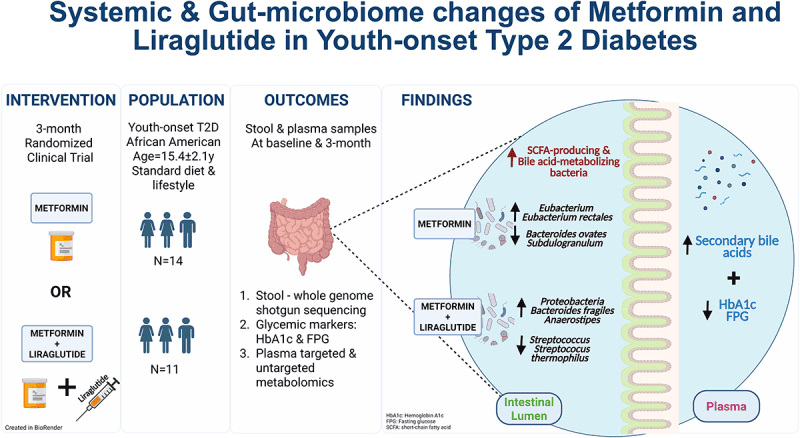
Graphical Representation
The First Colonizers: How Bifidobacterium breve Shapes the Infant Microbiome
The study dives into the genetics of Bifidobacterium breve, a key early-life gut microbe, and asks: what allows it to successfully colonize the infant gut and shape host-microbe interactions? Using a large-scale mutant library, the researchers identified hundreds of genes that matter for colonization in germ-free models. They found that many of these genes link directly to carbohydrate metabolism (especially human-milk-oligosaccharide and mucin-derived sugars), cell-surface structures like pili and exopolysaccharides, and molecules that might influence the host immune system. While this is largely a mechanistic and genetic study, the implications are clearly microbial-ecosystem-based.
Viewed through the microbiome lens, what stands out is how B. breve isn’t just a passive inhabitant—it actively competes, adapts and influences its niche. The ability to extract certain sugars, build adhesion/colonization machinery, and perhaps release small immunomodulatory compounds means it can shape the gut ecosystem, opening space for itself and modulating the host environment in tandem. That means when we see infants with higher Bifidobacterium levels, it may be in part because these colonization-traits are successful, and that success may ripple out to influence immune development, pathogen resistance, or later microbiome trajectories.
For a science-curious audience, this research signals that early-life microbial assembly isn’t a black box—it’s driven by specific microbial genes and traits. It invites us to think of the infant gut not just as a “microbe sandbox”, but as a competitive ecosystem where microbes like B breve bring their own toolkit—carbohydrate-foraging enzymes, adhesion molecules and immune-modulating products—and in doing so, set the stage for how the gut community and host interact for months or years to come.
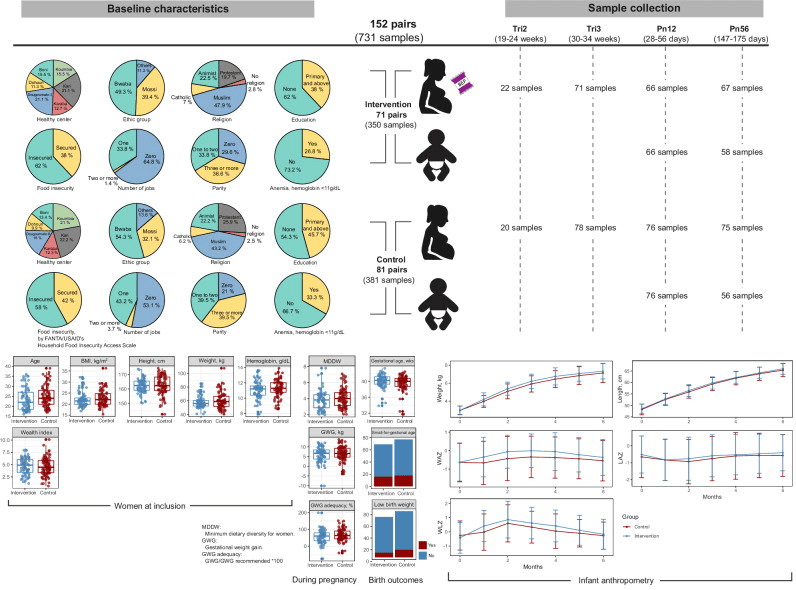
Pie charts depict the baseline characteristics of the study participants, including health center, ethnicity, religion, education level, food insecurity, number of jobs, parity, and anemia for each group. Box plots present other measures, such as the age of the participants, BMI, height, weight, hemoglobin levels, and wealth index, along with minimum dietary diversity score and gestational weight gain during pregnancy. Birth outcomes are displayed using box plots and bar plots. Infant anthropometry data are presented as growth curves for weight, length, and corresponding Z-scores (WAZ, LAZ, and WLZ) during the first six months of life, comparing the intervention and control groups. Box plots indicate median (middle line), 25th, 75th percentile (box), and the smallest and largest values within 1.5 × interquartile range (IQR) from the box (whiskers). Data points beyond this range are considered outliers (single points). Data in line plots are presented as mean ± standard error. BMI body mass index, GWG gestational weight gain, MDDW minimum dietary diversity for women, Pn12 postnatal 1–2 months, Pn56 postnatal 5–6 months, Tri2 trimester 2, Tri3 trimester 3, LAZ length-for-age Z-score, WAZ weight-for-age Z-score, WLZ weight-for-length Z-score. |
From Veggies to Vitality: The Microbes That Activate Broccoli’s Health Magic
When researchers tested a daily dose of a Broccoli Sprout Extract (BSE) rich in the bioactive compound sulforaphane in adults with pre-diabetes, they discovered that the gut microbiome turned out to be a crucial piece of the puzzle. Overall, the treatment trimmed fasting blood glucose by ~0.2 mmol/L—not quite hitting the target—but when they looked closer they found a subgroup of “responders” whose gut microbial community stood out.
What distinguished those responders was a higher abundance of a gene operon encoded by certain Bacteroides species (the so-called BT2160 regulator) which helps convert the sulforaphane precursor into its active form. Those with more of that operon had higher serum sulforaphane levels and stronger metabolic response. In addition, the microbiome profiles of responders had more of the beneficial butyrate-producing taxa (for example Faecalibacterium prausnitzii and Roseburia intestinalis) and fewer facultative anaerobes/ oral-pathogen linked members (like Veillonella and Streptococcus) than non-responders.
From a microbiome-perspective, this means the gut isn’t just a passive bystander in dietary interventions—it’s actively shaping how a bioactive compound is processed and how the host metabolism responds. The study suggests that two people with identical doses of BSE might have different outcomes because their gut microbial gene toolkit differs. It highlights that early-life or baseline microbiome composition can set the stage for whether an intervention “sticks”. In broader terms, it nudges us toward a future of precision nutrition where you might screen your gut microbes before you choose a supplement or dietary strategy—and where the success of interventions may depend as much on the microbes inside you as on the foods or extracts you ingest.
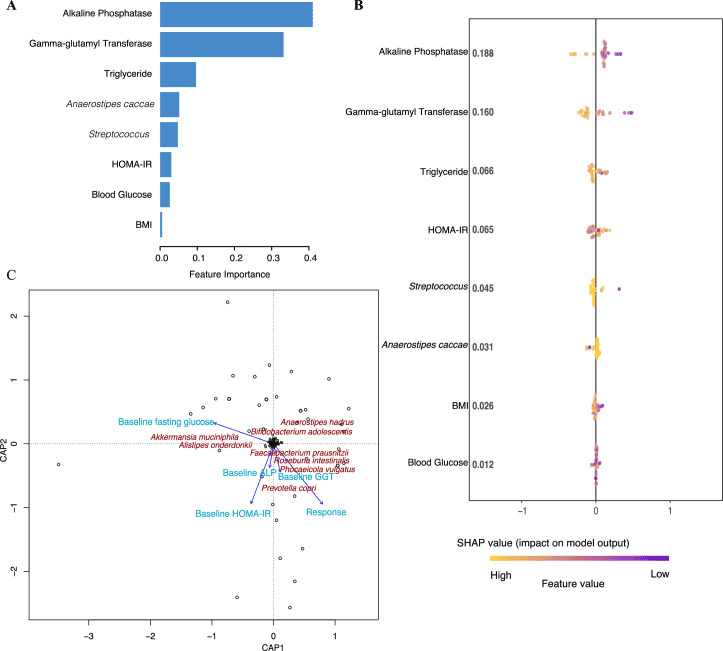
a) The relative importance of baseline variables (features) in predicting the change in fasting glucose after treatment with BSE. The measures of relative feature importance sum up to 1 and were generated by XGBoost. All participants receiving BSE were included in these analyses to investigate the importance of continuous baseline variables across all individuals. b) SHAP summary plot for the impact of baseline variables in predicting the change in fasting glucose in response to BSE treatment. Each point in the figure corresponds to a participant, and the SHAP value reflects the impact of the baseline variable in predicting the change in fasting glucose for that individual. For example, in the row corresponding to alkaline phosphatase, all individuals receiving BSE are plotted in accordance with how much baseline alkaline phosphatase predicts the change in fasting glucose. The panel is arranged based on the mean of the absolute SHAP values for each baseline variable. c) The impact of baseline clinical and microbiota variables on the glycemic response to BSE treatment using a distance‐based redundancy analysis model, calculated by the cap scale function in R (the vegan package with the significance level based on the anova.cca function and PERMANOVA test with 10,000 permutations; P = 0.1). BMI is body mass index, blood glucose denotes fasting blood glucose, ALT is alkaline phosphatase, GGT is gamma‐glutamyl transferase, and HOMA‐IR is the homeostasis model assessment‐2 estimate of insulin resistance. |
The Gut Connection: How Microbes Influence Immunotherapy Success in Lung Cancer
In a large biomarker study tied to the JCOG2007 (NIPPON) phase III trial in advanced Non‑small‑cell lung cancer (NSCLC), researchers asked whether the gut microbial community might help explain why some patients respond better — or suffer worse — when given first-line chemo-immunotherapy. From baseline stool samples of 270 patients, they looked at microbial diversity, community structure and the abundance of specific bacterial genera, and then linked those findings to overall survival and treatment-related serious side-effects.
What stands out from a microbiome perspective is that patients who lived longer (surviving beyond 12 or 18 months) tended to have higher relative abundance of genera such as Fusicatenibacter, Butyricicoccus and Blautia — all taxa previously associated with healthy gut ecosystems and short-chain fatty acid (SCFA) production.Conversely, a lower microbial alpha-diversity and presence of less favorable taxa (for example, Prevotella-family group NK3B31) correlated with higher mortality or greater risk of severe adverse events (grade 4+).Of particular interest: the prognostic associations were stronger in the subgroup receiving the dual immune checkpoint combination (nivolumab + ipilimumab + chemotherapy) than in the chemotherapy-plus-PD-1 monotherapy arm, hinting that the microbiome might modulate complex immune-chemotherapy interactions.
From a science-curious standpoint, this paper underscores how the gut microbiome is more than background noise in cancer therapy—it may act as a modulator of both efficacy and toxicity. The presence of SCFA-producers like Fusicatenibacter and Butyricicoccus could reflect a gut ecosystem better poised to support immune-effector functions, maintain gut barrier integrity, and reduce systemic inflammation. On the flip side, loss of diversity or enrichment of potentially dysbiotic taxa may tilt the immune-metabolic milieu toward less favourable outcomes. In short, the study invites us to think of first-line chemotherapy + immunotherapy not just as “drugs for human cells” but as treatments whose success may depend on the state of the microbial ecosystem inside us.
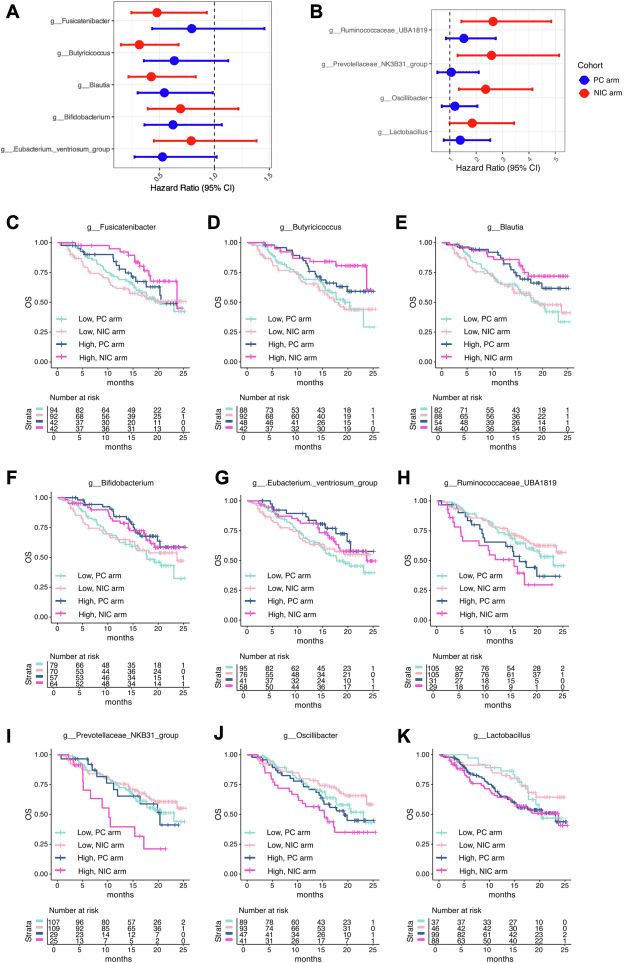
Association between gut microbiota composition and OS by treatment regimen. (A) HRs for OS associated with the abundance of specific bacterial genera in the PC arm. (B) HRs for OS associated with the abundance of specific bacterial genera in the NIC arm. (C–K) Kaplan-Meier survival curves for OS in patients with a high versus low abundance of key bacterial genera after stratification by treatment regimen: (C) Fusciantibacter, (D) Butyricicoccus, (E) Blautia, (F) Bifidobacterium, (G) Eubacterium ventriosum group, (H) Ruminococcaceae UBA1819, (I) Prevotellaceae NK3B31 group, (J) Oscillibacter, and (K) Lactobacillus. HR, hazard ratio; NIC, nivolumab and ipilimumab plus platinum doublet chemotherapy; OS, overall survival; PC, pembrolizumab plus platinum doublet chemotherapy. |
