- March 22, 2019
- Koushalya
- Microbiome and Drugs
Commensals and Cancer Therapy
In our previous blog on “commensals and cancers” we tried to compile the updated information on relationship between gut microbiome and its effect on cancer (in general) and colorectal cancer (CRC) (in specific). Dysbiosis of gut microbiome is one the best studied associations for colorectal cancer, apart from its genetic and lifestyle related triggers. The scientific community has already deemed that the next decade of research in the field of oncology will be dominated by greater integration of microbiome and its impact, which will drive the invention and discovery of novel microbiome derived therapeutics of cancer.
Given this context, cancer therapy has also gained momentum in the recent times, with reports of microbial communities within the gut modifying (both potentiating and weakening) the cancer drugs. This has started a dialogue on the need for in-depth knowledge of the impact of the microbiome on cancer therapeutic strategies.
Microbiome and Modulation of Cancer Therapy
Human microbiome, specifically the gut microbiome has been extensively reported to modulate antitumor efficacy of chemotherapies and immunotherapeutic agents, in many pre-clinical models. It achieves it so largely in two ways; “Modulate the immune system and Modify the drug or aid the process of such modifications”.
Through such modification the microbiome is said to either potentiate (increase) or weaken (decrease) the efficacy of immunotherapy and hence deemed essential for optimal drug efficacy of cancer drugs.
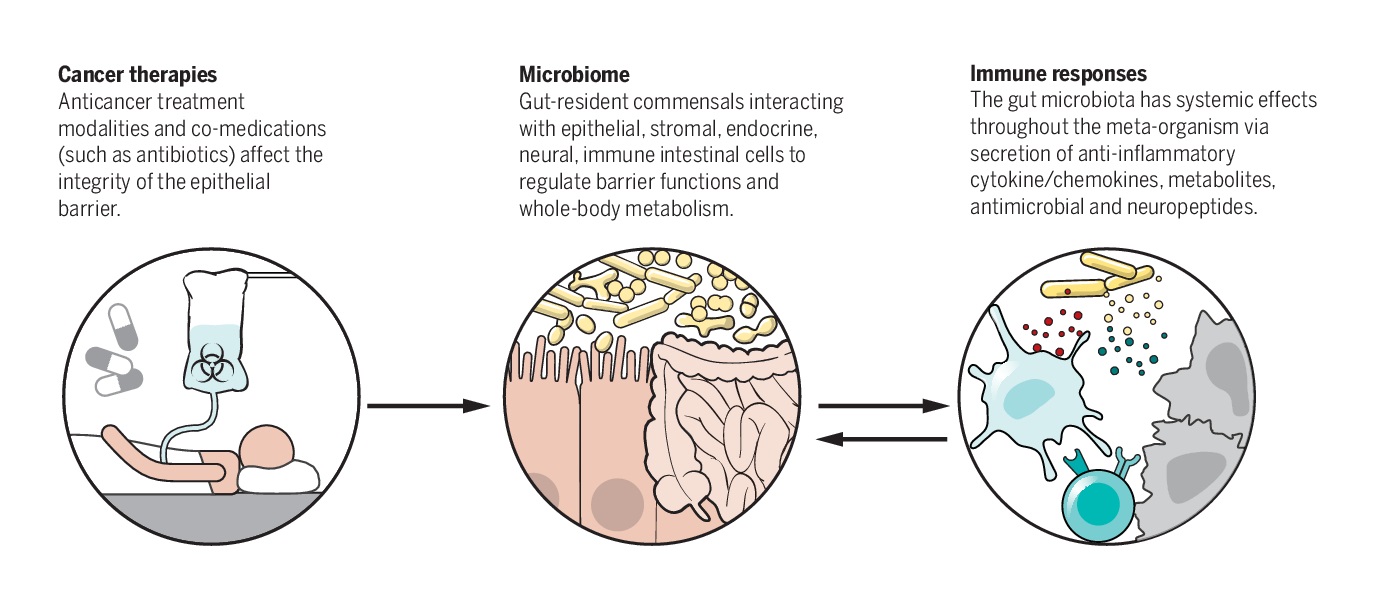
Modification of CRC Drug (5-FU)
Standard systemic treatment for CRC (metastatic and/or irresectable stage) is mostly based on fluoropyrimidine, such as 5-fluorouracil (5-FU). It is orally administered as capecitabine, or TAS-102. Capecitabine is a precursor of 5-FU, which must get converted to its active form to be effective against CRC. Many in vitro studies have indicated a significant role of gut microbiota in this conversion. An increased response of CRC cell lines to 5-FU in presence of Lactobacillus plantarum supernatant has been reported. 5-fluorocytosine (5-FC) samples, incubated with viable Escherichia coli showed a higher increase in active 5-FU concentration. 5-FC, incubated with human faecal samples, also displayed a significant degradation reaction, indicating a significant increase in its potentiation. Hence associated with efficacy of drug and response.
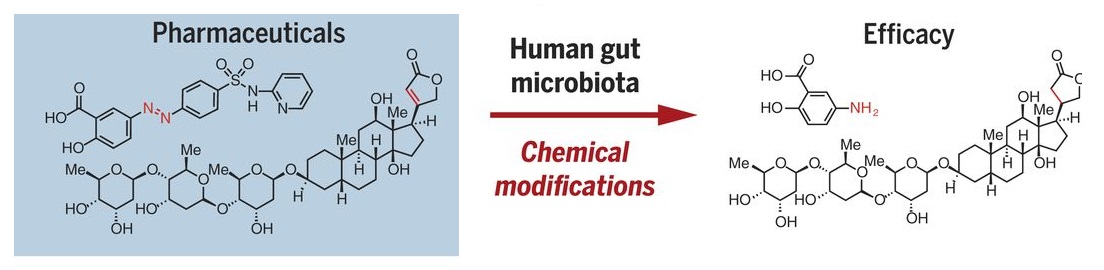
On the other end of the spectrum, Colorectal cancers enriched in Fusobacterium nucleatum have demonstrated worse prognosis. Because of the bacteria conveyed resistance to oxaliplatin and 5-fluorouracil by inducing autophagy. Oral administration of 5-Fu has been reported to alter the bacterial diversity and community composition of the gut. Several such instances have been reported involving both chemotherapeutic efficacy and chemotherapeutic cytotoxicity.
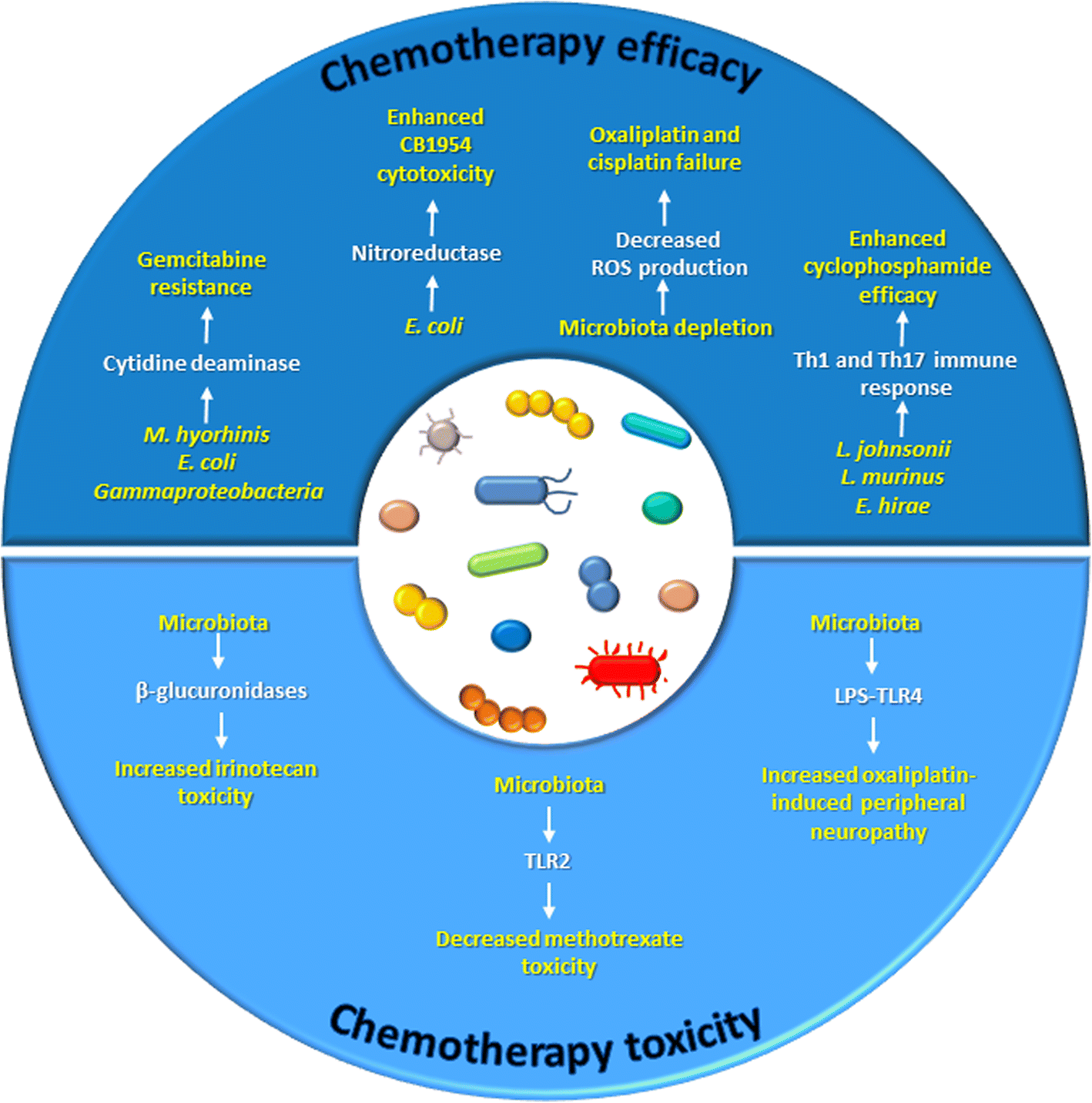
Microbiome and Modulation of the Immune System
Effector T lymphocytes represent a critical branch of the adaptive immune response to antigens. But, controlling the length and strength of this activation is very critical, which if unchecked can go damage even self-cells. Hence, a series of coinhibitory molecules, called immune checkpoints, are expressed by antigen presenting cells for switching off T cell activation.
Tumour cells inactivate cytotoxic CD8+ T cells, by expressing the same immune checkpoint molecules, and evade the anti-tumour immune response. Hence, targeting these immune checkpoints has emerged as a promising approach for cancer therapy. The most prominent one has been the class of drugs that act against programmed cell death protein 1 (PD-1) and PD-1 ligand 1 (PD L1).
However, patient responses to immune checkpoint therapies have been heterogeneous, varying with every individual and been reported to be transient. A cohort of 61 patients evaluated that PD-L1 expression in colorectal cancer defines three subsets of tumour immune microenvironments. Of which, third group, with low TANs, high IICs, both IICs and NCs PD-L1 expressing, would be the ideal candidate for this type of therapy. However, heterogeneous and transient responses to anti-PD1 therapy was still a concern.
In this context, studies have reported that the gut microbiota, possessing a pronounced modulatory effect on the immune system, may enhance responses to immune checkpoint therapies. Several Bifidobacteria species seem to stimulate dendritic cells (a kind of antigen presenting cells) in tumours, boosting the number of CTLs. This together have been reported to boost the response of anti-PD-1 and PDL1 drugs. This not only cleared the air about the previously observed heterogeneous and transient responses, but also established the impact of gut microbiome on cancer therapy.
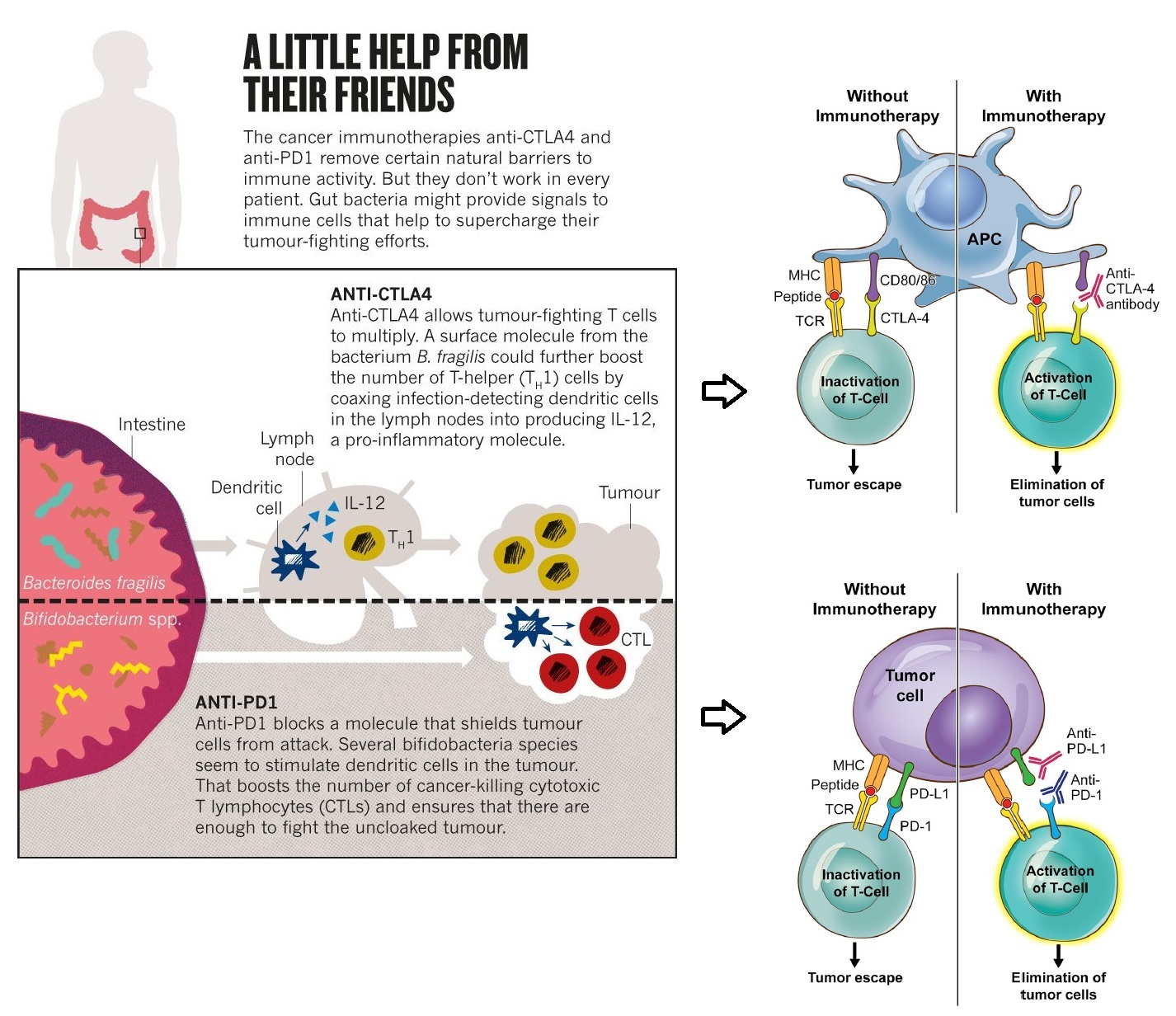
Faecal Microbiota Transplantation (FMT) and CRC Immunotherapy
By definition, "FMT involves administration of faecal material (containing intestinal microbiota) from a healthy individual (donor) to treat dysbiosis and restore beneficial intestinal flora and phylogenic diversity.” FMT has proven to be beneficial in the treatment of recurrent Clostridium difficile infection (CDI) and few other gastroenteric disorders.
Given that gut microbiome has such a significant on cancer therapy, it has recently found traction in cancer chemotherapeutic assistance. The transfer of highly complex whole communities of microorganisms from responders has been shown to result in durable changes in the recipient. FMT from colorectal patients into germ-free mice can elicit dysplasia and polyp formation. FMT from cancer patients responding to PD-1 based immunotherapies into germ free mice have shown increased efficacy. Non-responders to immune therapies were characterized by low abundance of Akkermansia muciniphila. Response to tumour therapy was improved by oral supplementation of the same bacterium.
Conclusion
The learning from these emerging studies, linking tumour development, progression, therapeutics and microbiome composition, is the fact that it is the microbiome which is key for developing personalized therapeutic approaches, since the microbiome composition of every individual is unique. At the same time, the shear complexity of the microbiome also adds several layers of complications to the precision medicine paradigm, but will no doubt drive the development of future therapeutics in oncology and other medical disciplines. The vision of utilizing a panel of bacterial marker species, that would designate an individual to be a responder or non-responder, is not too far from implementation, and will be checked before by clinicians to decide on personalized therapeutic approach. Overall, it is evident that the composition and diversity of the intestinal microbiota has significant implications in cancer treatments but will have to be thoroughly evaluated before implementation.