- June 27, 2023
- Shruti Garg
- Microbiome and Disease
Gut Microbiome in Cancer: Exploring the Latest Developments
The gut microbiome has emerged as a crucial player in various aspects of human health, including cancer development and progression. The trillions of microorganisms inhabiting the human gut play an essential role in modulating the immune system and metabolism. Recent research has uncovered fascinating insights into the complex interplay between the gut microbiome and cancer, thereby opening new avenues for targeted therapies and preventative measures. This article delves into the latest findings in gut microbiome research, highlighting key areas of interest and potential clinical applications.
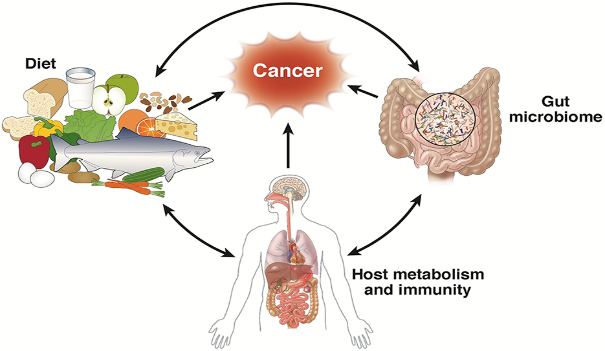
1. The Role of Gut Microbiome in Cancer Development
1.1 Microbial Dysbiosis and Inflammation:
Chronic inflammation is a well-known risk factor for various cancers, including colorectal cancer. Emerging evidence suggests that an imbalance in the gut microbiota composition, known as dysbiosis, may contribute to chronic inflammation and cancer development. Dysbiotic gut microbiota can stimulate pro-inflammatory immune responses, leading to DNA damage and increased susceptibility to cancer. Moreover, certain pathogens such as Helicobacter pylori and Fusobacterium nucleatum have been implicated in promoting inflammation and tumour growth.
1.2. Genotoxic Bacterial Metabolites:
Some gut bacteria produce genotoxic metabolites that can directly damage host DNA, increasing the risk of cancer. For example, Escherichia coli strains carrying the pks pathogenicity island produce a genotoxin called colibactin, which induces DNA double-strand breaks in host cells. Similarly, the Cytolethal Distending Toxin (CDT) produced by certain Proteobacteria can cause genomic instability and promote tumorigenesis.
1.3. Altered Metabolism and Cancer Risk:
The gut microbiome plays a significant role in modulating host metabolism, and alterations in microbial metabolic pathways have been linked to cancer risk. For instance, gut bacteria can convert dietary components such as red meat and dietary fibres into potentially carcinogenic or tumor-suppressive metabolites, respectively. High consumption of red meat has been associated with an increased risk of colorectal cancer, partly due to the production of harmful secondary bile acids and genotoxic N-nitroso compounds by gut bacteria.
2. Gut Microbiome and Cancer Therapy
2.1. Impact on Immunotherapy:
Recent studies have shown that the gut microbiome can influence the effectiveness of cancer immunotherapy, particularly immune checkpoint inhibitors. Specific bacterial species, such as Bacteroides fragilis and Enterococcus hirae, have been found to enhance the antitumor efficacy of immune checkpoint inhibitors by modulating immune cell activity and promoting a favourable immune response against tumors. Thus, manipulating the gut microbiota may offer a novel strategy to improve the effectiveness of cancer immunotherapy.
2.2. Influence on Chemotherapy:
Gut microbiota can also impact the host response to conventional chemotherapeutic drugs. For example, the anticancer effects of the platinum chemotherapeutic oxaliplatin and the alkylating agent cyclophosphamide are attenuated in germ-free or antibiotic-treated mice, highlighting the importance of gut microbiota in mediating the therapeutic response to these drugs. Furthermore, targeting specific bacterial enzymes, such as β-glucuronidases, may help reduce chemotherapy-induced toxicity and improve treatment outcomes.
3. Targeting the Gut Microbiome for Cancer Prevention and Treatment
3.1. Probiotics and Prebiotics:
The use of probiotics (beneficial live bacteria) and prebiotics (non-digestible fibers that promote the growth of beneficial bacteria) has gained considerable attention as a potential strategy to modulate the gut microbiome and reduce cancer risk. Some studies have shown that the consumption of probiotics and prebiotics can enhance the production of short-chain fatty acids, such as butyrate, which exhibit tumor-suppressive effects. Moreover, specific probiotic strains, such as Lactobacillus spp., have been found to improve immune function and reduce inflammation, potentially reducing the risk of cancer.
3.2. Fecal Microbiota Transplantation (FMT):
FMT involves transferring the fecal microbiota from a healthy donor to a patient with a dysbiotic gut microbiome, aiming to restore eubiosis and improve health outcomes. While FMT has shown promising results in treating recurrent Clostridium difficile infection, its potential application in cancer prevention and treatment is still under investigation. Some preclinical studies suggest that FMT may help reduce inflammation and improve the response to cancer therapies; however, more research is needed to establish the safety and efficacy of this approach in cancer patients.
3.3. Personalized Microbiome-based Interventions:
Given the inter-individual variability in gut microbiome composition and response to therapies, personalized microbiome-based interventions hold promise for improving cancer prevention and treatment outcomes. By integrating information on an individual's gut microbiota composition, genetic background, and environmental factors, personalized strategies, such as targeted dietary modifications, probiotics, or prebiotics, could be developed to modulate the gut microbiome and reduce cancer risk or enhance treatment response.
4. Challenges and Future Directions
Despite the significant progress made in understanding the role of the gut microbiome in cancer, several challenges remain. These include:
4.1. Causality and Mechanistic Insights:
Establishing causality between gut microbiota alterations and cancer development is challenging, as most studies are observational and cannot distinguish between cause and effect. Moreover, understanding the complex mechanisms by which gut microbiota influence cancer risk and therapy response requires further research using advanced experimental models and multi-omics approaches.
4.2. Standardization of Methodologies:
There is a need for standardized methodologies in gut microbiome research, including sample collection, processing, sequencing, and data analysis. This will facilitate the comparison of results across studies and enable the identification of consistent patterns and biomarkers associated with cancer risk and treatment response.
4.3. Clinical Translation:
Translating findings from preclinical studies to clinical practice is a major challenge, as many factors can influence the gut microbiome and its interaction with the host, including diet, lifestyle, genetics, and environmental exposures. Large-scale, well-designed clinical trials are needed to establish the safety and efficacy of microbiome-based interventions in cancer prevention and treatment.
Conclusion
In conclusion, the gut microbiome plays a pivotal role in cancer development and therapy response. Further research is needed to unravel the complex interplay between gut microbiota, host factors, and environmental exposures, and to develop effective microbiome-based strategies for cancer prevention and treatment. With advances in sequencing technologies, bioinformatics, and experimental models, the field of gut microbiome research holds immense potential for improving our understanding and management of cancer.