Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
A Microbial Fingerprint in Parkinson’s: The Imidazole Propionate Connection
The gut microbiome is increasingly being implicated in brain health, and this new study uncovers a striking connection between a common bacterium and Parkinson’s disease. Researchers discovered that Streptococcus mutans, usually known for its role in dental cavities, is enriched in the guts of Parkinson’s patients—especially strains carrying a gene called urdA. This gene enables the production of a metabolite, imidazole propionate (ImP), which turned out to be more than just a byproduct of microbial metabolism.
By analyzing nearly 500 human metagenomes, the team found a consistent signal: Parkinson’s patients had higher levels of S. mutans harboring urdA, while other histidine-related pathways remained unchanged. To probe causality, they introduced the bacterium into germ-free mice. Animals colonized with urdA-positive S. mutans, or even with E. coli engineered to express urdA, showed increased ImP in blood and brain, developed loss of dopamine neurons in the substantia nigra, and displayed motor impairments. Strikingly, pasteurized bacteria or strains without urdA had no such effect.
The mechanism pointed to the mTORC1 signaling pathway. ImP activated mTORC1 inside dopaminergic neurons, setting off a cascade that led to cell stress and death. Blocking this pathway with rapamycin largely protected neurons, reducing both neuroinflammation and motor symptoms, even though ImP levels remained high.
In patient samples, plasma ImP was also elevated, tying the microbial signal back to humans. This paints a compelling picture of a gut–brain axis where a specific microbial gene and metabolite may help drive neurodegeneration. From a microbiome perspective, it highlights how small shifts in gut composition—particularly the rise of urdA-positive S. mutans—could have outsized impacts on brain health, and opens the door to therapies aimed at microbial metabolism or its signaling consequences.
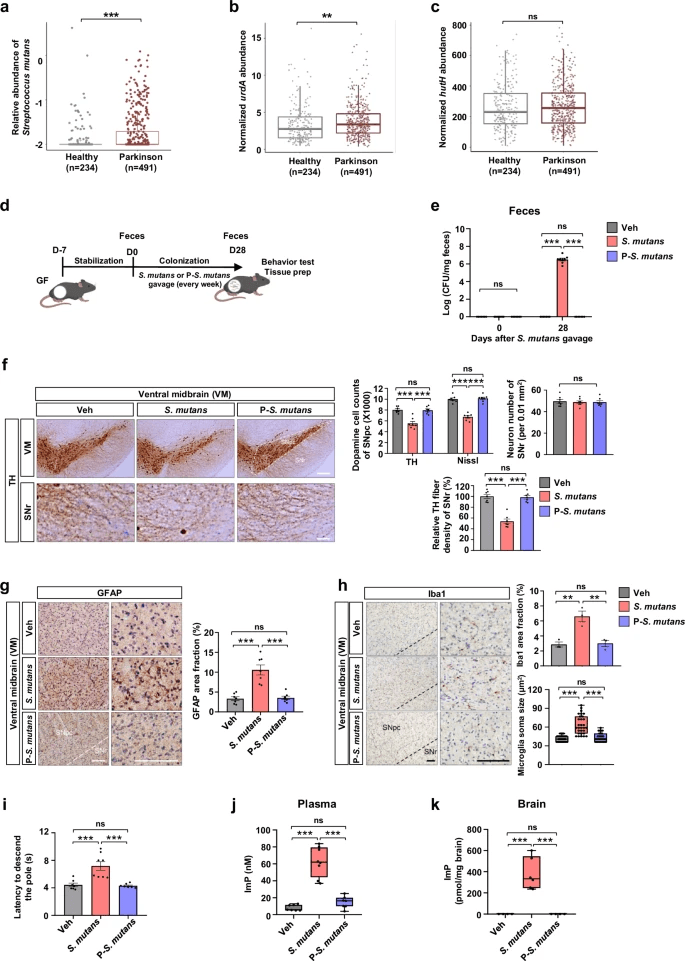
Increased urdA enzyme abundance in the gut microbiome of patients with Parkinson’s disease (PD) and PD key pathologies induced by gut-colonization of imidazole propionate (ImP)-producing Streptococcus mutans (S. mutans).
From Microbes to Mobility: The Gut–Brain Link in Hydrocephalus
In a recent shotgun metagenomics study, researchers explored how the gut microbiome in idiopathic normal pressure hydrocephalus (iNPH) differs from that of healthy older adults and people with enlarged brain ventricles but no symptoms. iNPH, known for causing ventricle enlargement, problems walking, dementia, and incontinence, may be linked to gut-brain interactions, including inflammation and changes in cerebrospinal fluid dynamics.
Compared to healthy controls with normal ventricles (nvHC), both the iNPH group and the enlarged ventricles controls (evHC) showed shifts in their microbial communities. Species previously connected to disease states — notably Enterocloster bolteae and Ruminococcus gnavus — were enriched in iNPH, while taxa often associated with gut health — including Ruminococcus lactaris and Fusicatenibacter saccharivorans — were depleted. Some bacteria, such as Evtepia gabavorous, Cuneatibacter sp., Anaerotruncus massiliensis, and Eisenbergiella tayi, were particularly associated with clinical features of iNPH, especially measures like ventricular enlargement and gait impairment.
On the functional side, the gut microbiomes of the iNPH and evHC groups showed elevated potential for certain metabolic pathways: ones related to carbohydrate and amino acid metabolism, especially S-adenosyl-L-methionine (SAM) and methionine biosynthesis. These pathways are suggestive of immune or epigenetic effects, which could influence inflammation or neurochemical signaling in the brain. Although markers of intestinal inflammation (like calprotectin) didn’t differ significantly between groups, there were positive correlations between calprotectin levels and disease-associated bacterial species, and negative correlations with health-linked ones.
Altogether, the findings point to a gut microbiome “signature” in iNPH: not just general dysbiosis, but specific species and functional pathways that track with structural brain changes and mobility issues. The study suggests that some microbiome changes precede or accompany the physical progression of ventriculomegaly, possibly contributing via metabolic, immune, or barrier-related mechanisms. Tracking how the “enlarged ventricle but no symptoms yet” group’s microbiome moves over time may help indicate whether these microbial shifts are cause, consequence, or both — but either way, they may offer biomarkers or therapeutic entry points.
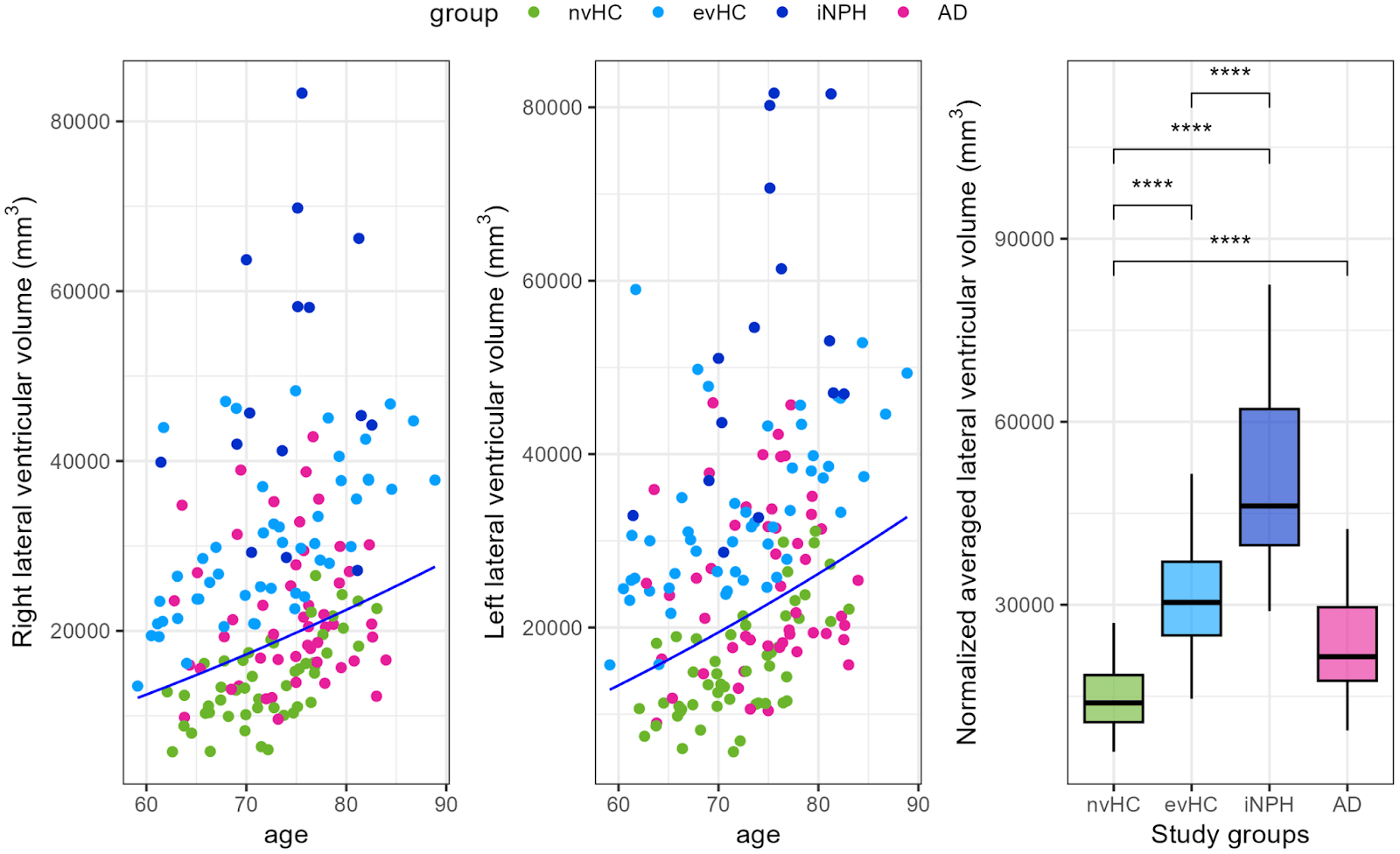
Brain ventricular volumes by study group. The left and right lateral ventricular volume of evHC group participants lies above the 80th percentile of the ADNI cohort’s normal distribution, represented by a dark blue line (A,B). The study groups differ significantly in brain ventricular volume (C). Statistics: Wilcoxon rank-sum test with **** p < 0.0001.
Longevity and the Microbiome: Secrets from the Oldest-Old
The human gut undergoes remarkable shifts with age, and a recent study of long-lived individuals in China offers new insight into how microbial communities evolve from the early 60s to nearly 100 years of age. By sequencing the gut microbiome of healthy adults grouped into younger-old (60–74), middle-old (75–89), and oldest-old (90–99), researchers uncovered a dynamic picture of decline, loss, and unexpected resilience in the microbial world that accompanies aging.
One of the most striking patterns was a decline in overall diversity from the younger-old to the middle-old group, followed by a partial rebound in the oldest individuals. This suggests that longevity may be linked to a microbiome capable of reorganizing or re-stabilizing after midlife decline. Specific species told more of the story: Bacteroides stercoris, abundant in younger participants, steadily declined with age, along with pathways for lipopolysaccharide biosynthesis. Meanwhile, Prevotella stercorea was enriched in the younger-old group and tied to energy-generating pathways like oxidative phosphorylation.
As age advanced, species such as Clostridium sp. CAG:169, Enterocloster lavalensis, and Enterococcus faecalis became more prominent. Some of these increases were accompanied by a rise in antibiotic resistance genes, including those linked to vancomycin resistance, particularly in the oldest-old group. Functional pathways showed similar non-linear shifts, with metabolism of amino acids and xenobiotics dipping in the middle years but re-emerging in extreme old age. Even archaea such as Methanobrevibacter smithii appeared as consistent players across all age groups, hinting at a stable but underexplored role in the aging gut ecosystem.
Rather than showing a simple decline, the findings reveal that the microbiome of healthy centenarians may adopt a unique balance: reducing some pro-inflammatory or pathogenic species while regaining metabolic functions that support resilience. From a microbiome perspective, this rebound pattern suggests that the key to longevity might lie not just in diversity, but in the gut’s ability to adapt, reorganize, and preserve crucial microbial functions deep into old age.
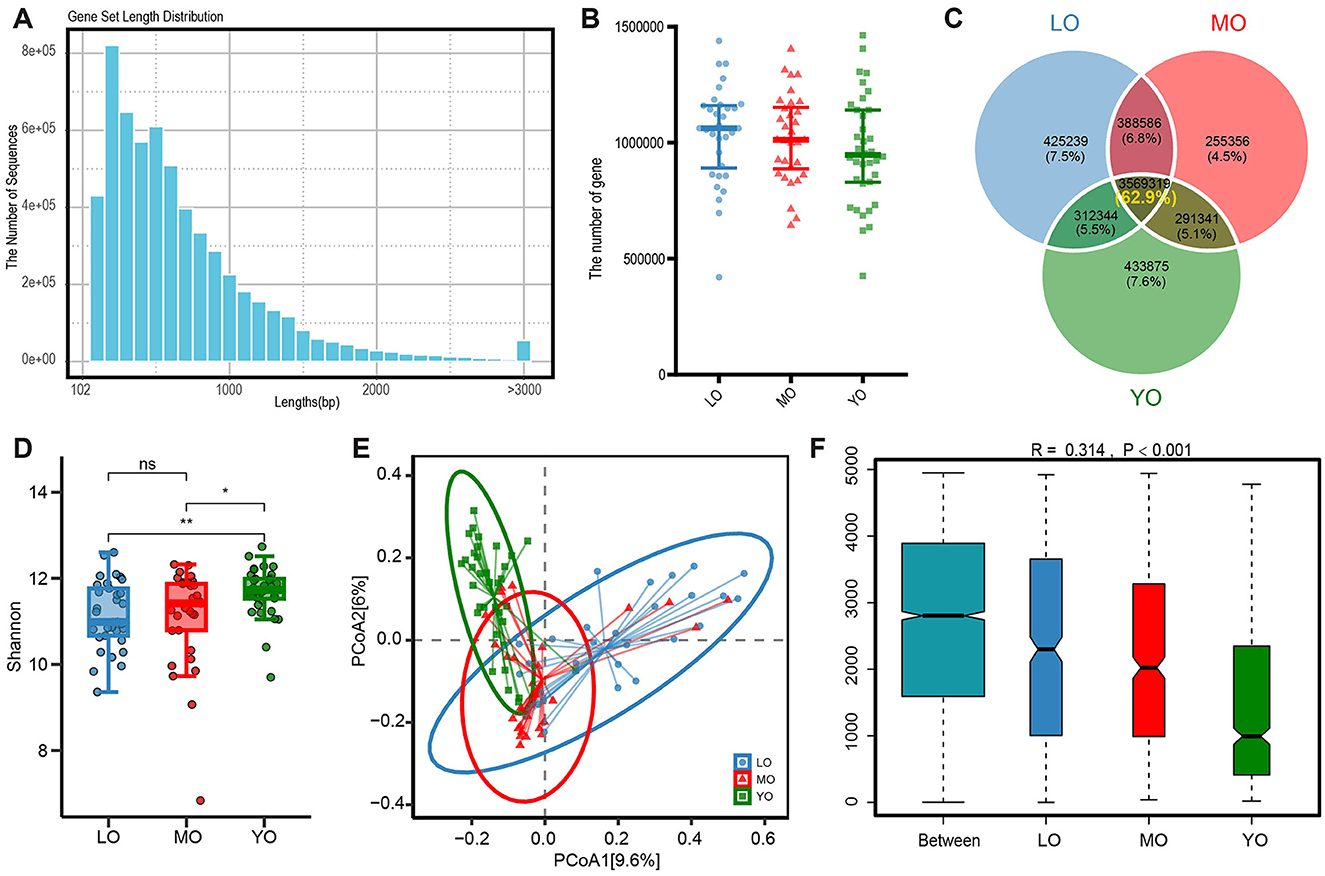
Outcomes of gene annotation analysis derived from metagenomic sequencing of the gut microbiome. (A)The histogram illustrates the distribution of lengths within the non-redundant gene set. (B) The scatter plot illustrates the differences in the quantity of non-redundant genes among the three groups. (C) The Venn diagram depicts the quantity and proportion of both shared and unique genes across the three sample groups. (D) The comparison of alpha diversity was conducted using the Shannon index. Each data point corresponds to a sample, while the box plot illustrates the median, quartiles, and outliers. ns, p-value > 0.05; *, p-value ≤ 0.05; **, p-value ≤ 0.01. (E) The analysis of β diversity was conducted using the Bray-Curtis distance metric. The principal coordinate analysis (PCoA) plot illustrates the distribution of gene composition across the three sample groups. (F) The ANOSIM (Analysis of Similarity) analysis of gut microbiota was conducted using the Bray-Curtis distance metric. The x-axis displays all samples categorized by group, while the y-axis illustrates the rank of the Bray-Curtis distance. The R is between (−1,1): if R > 0, the inter-group difference is significant; if R <0, the intra-group difference is greater than the inter-group difference. The reliability of the statistical analysis is represented by the p-value, with p-value <0.05 denoting statistical significance.
When Oral Bacteria Invade the Gut: Clues to Poor Sleep in Cancer Patients
In this study, researchers explored how sleep quality relates to the gut microbiome in people with colorectal cancer (CRC) and their cohabiting caregivers, teasing out microbial signatures that may connect sleep to gut health. They collected stool samples and detailed sleep diaries from 20 patient–caregiver pairs, and performed shotgun metagenomic sequencing to profile species-level microbiomes and functional potentials.
First off, the gut microbiomes of CRC patients displayed noticeable differences from those of their caregivers: patients had lower microbial diversity (by the Inverse Simpson metric) and a distinct overall compositional structure (beta diversity) . Among the 139 species-level taxa analyzed, 13 were differentially abundant between patients and caregivers. Beneficial taxa such as Coprococcus catus and Gemmiger formicilis were depleted in patients, whereas species like Clostridium clostridioforme and Blautia coccoides were more abundant in patients. Of particular note, Faecalibacterium prausnitzii, a well-known anti-inflammatory gut bacterium, was significantly lower in patients compared to caregivers.
Within the patient group, sleep efficiency (i.e. how well one stays asleep while in bed) showed intriguing links to microbial patterns. Patients with high sleep efficiency (≥ 85 %) had higher microbial diversity and lower abundances of certain oral-origin bacteria such as Actinomyces oris, Streptococcus mitis, and Atopobium species—suggesting that oral microbes translocated into the gut might influence sleep or that sleep quality constrains them. In contrast, taxa positively correlated with better sleep included Dorea formicigenerans and Lactobacillus rogosae, both of which have been implicated in favorable gut metabolic or fermentative roles.
Functional pathway analysis also hinted at links to sleep: for example, sleep onset latency correlated (negatively) with menaquinol (vitamin K₂) biosynthesis pathways, and time in bed correlated with pathways such as pyruvate fermentation to butanoate. While causality can’t be claimed here, these microbiome-sleep associations in cancer patients point toward a fascinating possibility: that shifts in gut’s microbial community—especially a rise in oral-derived species, loss of key gut-friendly taxa, and altered metabolic potentials—might weave into sleep health, perhaps via immune, metabolic, or inflammatory axes.
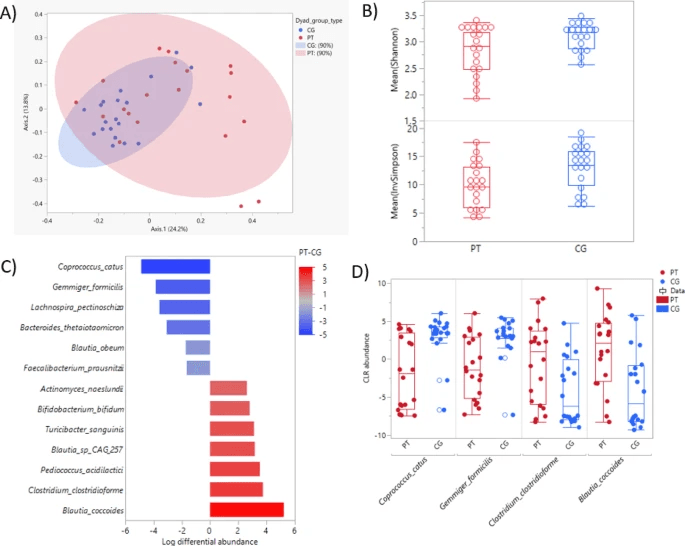
Gut microbiome features of caregivers and patients. (A) Principal Coordinate Analysis based on Bray–Curtis dissimilarity of gut compositional differences (R-squared: 0.070, p = .005). (B) Box-and-whisker plot of alpha diversity measured by Shannon Diversity Index (p = .058) and Inverse Simpson Diversity Index (p = .030) between patients and caregivers. (C) Differentially abundant taxa between patients and caregivers by log differential abundance (x-axis) (p < .05 and 20% FDR). Heat map gradient indicates log fold change difference. (D) Box plot of the two most abundant species and the two least abundant species in patients. Red: patients (PT); Blue: caregivers (CG).
Microbes, Metabolites, and the Mind: The Gut–Brain Link in Autism
Autism research is beginning to look beyond genes and the brain itself, turning instead to the gut for answers. This study highlights how specific microbial products from the tryptophan pathway may influence brain function and even shape the sensory and social experiences of children with autism spectrum disorder (ASD). By examining stool metabolites alongside brain imaging and behavioral measures, the researchers uncovered a striking link between gut chemistry and neurological outcomes.
Children with ASD showed lower levels of several tryptophan-derived compounds, including kynurenate and indole lactate. These molecules are produced when gut microbes metabolize dietary tryptophan, and they don’t just stay in the gut—they can enter circulation and interact with the brain. Kynurenate, for instance, is thought to be neuroprotective, and its depletion could leave neural circuits more vulnerable. Indole-based metabolites, many of which come from familiar gut commensals, were also altered, hinting that subtle microbial imbalances may reshape key chemical pathways.
These shifts were not random: they mapped onto differences in brain activity within the insula and cingulate cortex, regions involved in processing internal sensations, emotion, and social cues. Lower levels of certain indoles were tied to exaggerated brain responses to disgust or sensory input, while tryptophan betaine tracked with altered activity during social perception tasks. In turn, these neural signatures mediated links between metabolite levels and autism symptom severity, bridging gut chemistry and behavior.
From a microbiome perspective, the study points to a gut–brain pathway that is highly specific, involving microbial tryptophan metabolism feeding into neuroactive signals. Rather than a vague concept of “gut imbalance,” it suggests precise microbial metabolites that could serve as biomarkers of risk or resilience in ASD, and possibly targets for therapies that aim to restore balance in the gut–brain dialogue.
