Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.

Functional impact of microplastics on the human gut microbiome
This study investigates how exposure to microplastics affects the functional activity of the human gut microbiome. While previous research has mostly focused on changes in microbial composition, this study emphasizes the potential for microplastics to alter what gut microbes do—specifically their gene expression related to virulence, communication, transport, and plastic degradation. Researchers collected blood and stool samples from 39 healthy adults in Zhejiang Province, China. Using pyrolysis-gas chromatography/mass spectrometry, they identified five types of microplastics in blood: polyvinyl chloride, polyethylene, polypropylene, polystyrene, and polyamide 66. Participants were grouped by high and low microplastic exposure.
Shotgun metagenomic sequencing of stool samples showed that individuals with higher microplastic levels had gut microbiota with increased expression of genes involved in quorum sensing, virulence factors, transporter systems, and enzymes capable of degrading microplastics. Notably, while the microbial species composition did not change significantly, their functional profiles did, suggesting that microplastics influence microbial behaviour rather than which species are present.
To support these findings, mice were exposed to polystyrene microplastics over 14 weeks. Their gut microbiomes also exhibited similar functional shifts, reinforcing the link between microplastic exposure and altered microbial activity. Overall, the study highlights that microplastic exposure may not drastically shift the gut microbiota composition, but it can significantly impact microbial functionality. These functional disruptions could play an important role in health risks associated with microplastics, advancing our understanding of how environmental pollutants affect the microbiome and, potentially, human disease.
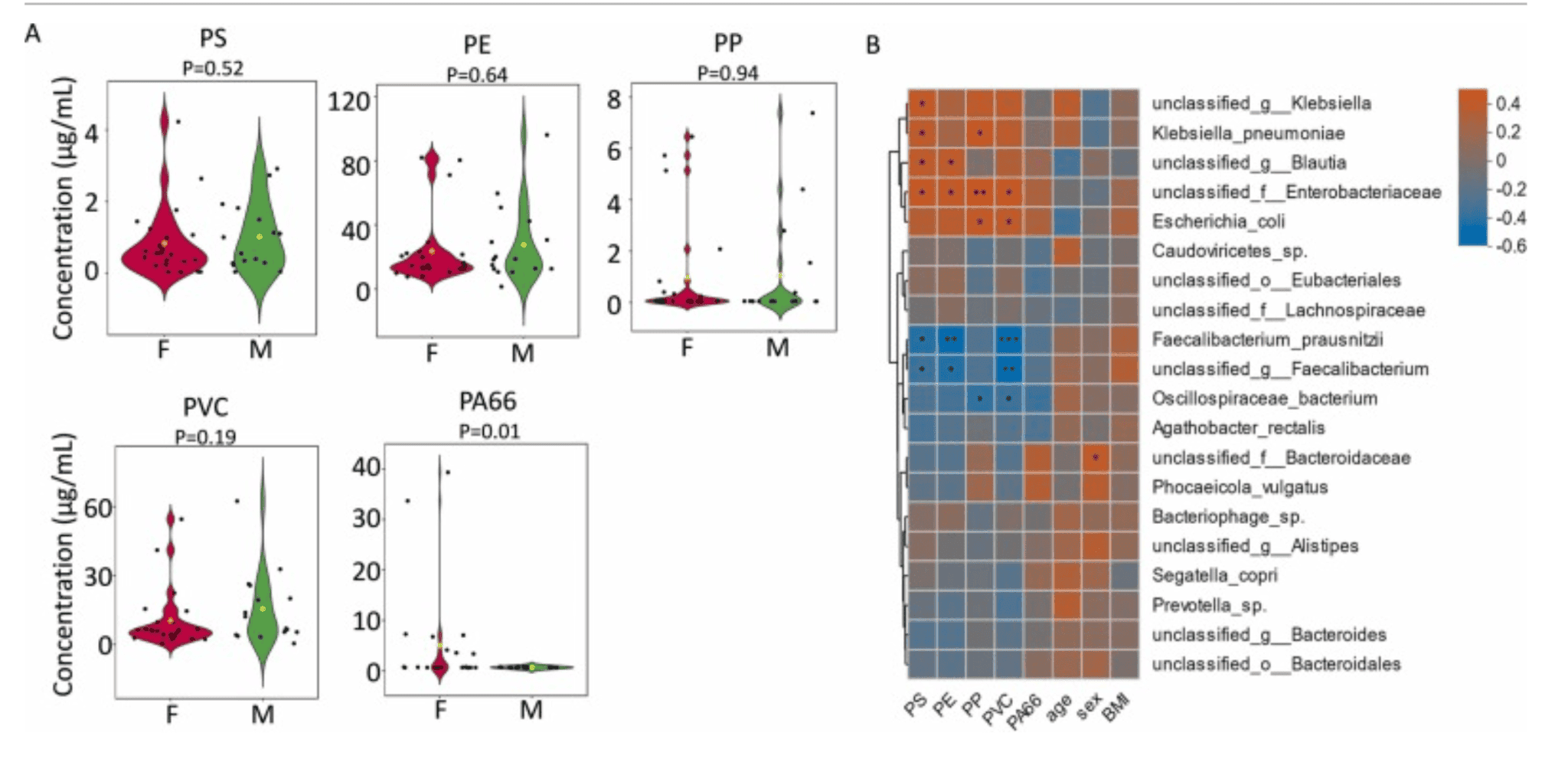
Association between microplastics and gut microbial species. (A) Concentration of five types of microplastics in human blood. (B) Spearman correlation between microplastics and top 20 abundant gut microbial species. * : p-value < 0.05; * *: p-value < 0.01; * ** : p-value < 0.001. |
Diet-driven microbiome shifts improve cardiometabolic outcomes
This study examines how adopting a non-industrialized, high-fibre, low-fat diet affects cardiometabolic and microbiome-related health in humans. Conducted as a randomized controlled trial, participants were divided into two groups: one consumed a traditional, fibre-rich diet similar to that of non-industrialized populations, while the control group continued with a typical industrialized Western diet. The primary aim was to determine how diet-driven changes in the gut microbiome could influence metabolic and cardiovascular health outcomes.
The results showed that individuals who switched to the non-industrialized diet experienced significant improvements in metabolic markers, including reductions in body weight, insulin resistance, and blood lipid levels. These physiological improvements were closely associated with notable changes in the gut microbiota. Specifically, there was an increase in microbial diversity and the abundance of beneficial fibre-degrading bacteria, such as Prevotella. Additionally, microbial metabolic pathways shifted toward enhanced production of short-chain fatty acids (SCFAs), which are known to support metabolic health and reduce inflammation.
The discussion emphasizes that the Western diet, typically low in fibre and high in processed fats and sugars, leads to a loss of beneficial microbial functions over time. The study supports the idea that restoring ancestral dietary patterns—even over a short duration—can reverse some of these effects. It underscores the strong connection between diet, gut microbiome composition, and cardiometabolic health. Overall, the findings advocate for dietary interventions that emphasize whole foods and fibre to re-establish beneficial host-microbe interactions and improve health outcomes.
Source: https://www.sciencedirect.com/science/article/pii/S0092867424014776?via%3Dihub
Psychosocial interventions reshape the gut microbiome and immune system
This study investigates how an immersive psychosocial intervention influences the human gut microbiome and immune system. Conducted as a community-based observational study, it involved 51 participants with an average age of approximately 50 years. The intervention was a nine-day inquiry-based stress reduction program designed to alleviate psychological stress
Researchers collected a total of 142 multi-omics samples from participants at three time points: before the intervention, during the program, and three months afterward. These samples were analysed to assess changes in the gut microbiome composition and cytokine levels, which are indicators of immune system activity. The study aimed to explore the complex associations between psychosocial interventions, gut microbiome dynamics, and immune responses.
Findings revealed that the psychosocial intervention led to significant shifts in the gut microbiome, including increased microbial diversity and alterations in specific bacterial populations. Concurrently, changes in cytokine profiles suggested modulations in immune function. These results indicate that psychological stress reduction can have measurable effects on both gut microbial communities and immune system parameters.
The study underscores the interconnectedness of psychological well-being, gut microbiota, and immune health. It suggests that interventions targeting psychological stress may beneficially influence gut microbiome composition and immune function, highlighting the potential of psychosocial strategies in promoting overall health.
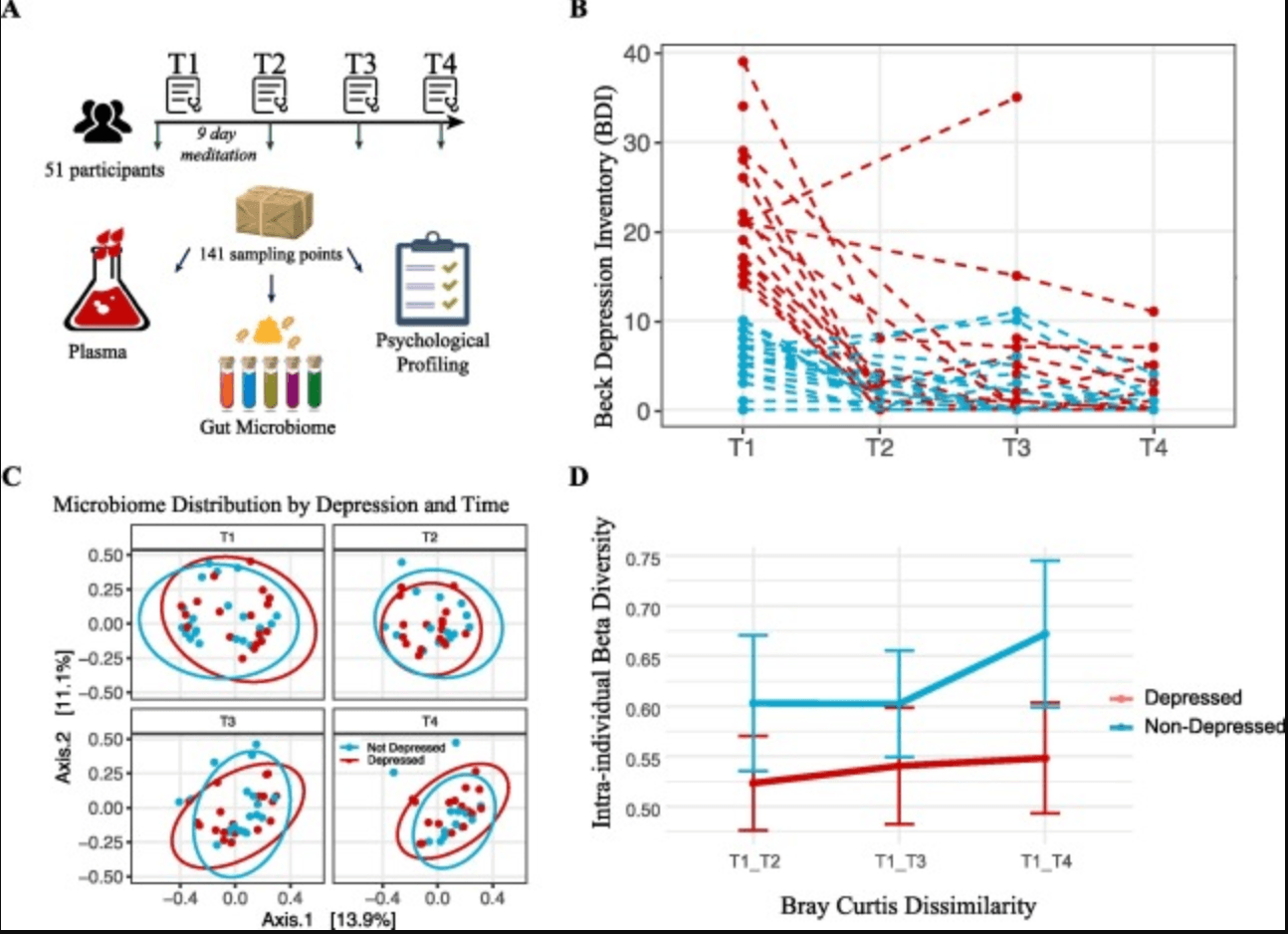
Study Design, Depression Trajectories, and Microbiome Composition Analysis. (A) Cartoon representation of the study's methodology (Image adapted from Pixabay). (B) Beck Depression Inventory-II (BDI-II) scores of participants across four time points, with each dashed line corresponding to one individual. The colour coding indicates whether the individual was depressed (red) or non-depressed (blue) at baseline. (C) Principal Coordinates Analysis (PCoA) of microbiome composition at the four time points. The first two axes are plotted, with the variance captured annotated along each axis. Red and blue colours denote the initial depression status of the participants, with red indicating depressed and blue indicating non-depressed individuals. (D) Intra-individual dissimilarity between T1 and the rest of the program. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) |
Omega-3 fatty acids improve lipid metabolism and gut microbiota in type 2 diabetes
The study titled "Impact of omega-3 fatty acids on hypertriglyceridemia, lipidomics, and gut microbiome in patients with type 2 diabetes" investigates the effects of omega-3 fatty acid supplementation on triglyceride levels, lipid profiles, and gut microbiota composition in individuals with type 2 diabetes. Conducted as a randomized controlled trial, the research aims to elucidate the potential benefits of omega-3 fatty acids in managing hypertriglyceridemia—a common lipid disorder in type 2 diabetes patients.
Participants were administered omega-3 fatty acids over a specified period, with assessments conducted before and after the intervention. The study measured changes in serum triglyceride levels, performed comprehensive lipidomic analyses to profile lipid species alterations, and analysed faecal samples to determine shifts in gut microbiota composition. These multifaceted evaluations aimed to provide a holistic understanding of how omega-3 supplementation influences metabolic and microbial parameters in the context of type 2 diabetes.
Results demonstrated that omega-3 fatty acid supplementation led to significant reductions in serum triglyceride levels, indicating an improvement in lipid metabolism. Lipidomic analyses revealed favourable changes in lipid species associated with cardiovascular health. Moreover, the intervention induced notable alterations in the gut microbiota, including increased diversity and the enrichment of beneficial bacterial taxa. These microbial changes are suggestive of enhanced gut health and potential anti-inflammatory effects.
In conclusion, the study provides evidence that omega-3 fatty acid supplementation can beneficially modulate lipid profiles and gut microbiota composition in patients with type 2 diabetes. These findings support the therapeutic potential of omega-3 fatty acids as an adjunctive treatment strategy for managing hypertriglyceridemia and improving metabolic health in this population.
Source : https://www.sciencedirect.com/science/article/pii/S0891422225000873?via%3Dihub
Gut microbiome alterations in ADHD and ASD : Role of probiotic interventions
This randomized controlled trial explored differences in gut microbiota between children with Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD), and evaluated the impact of probiotic supplementation on these microbial profiles. Initial analysis revealed distinct microbiome signatures in each group, with children with ASD exhibiting different bacterial compositions compared to those with ADHD. These differences suggest that gut microbiota may play a role in the unique behavioural and cognitive features of each disorder.
Participants were then given probiotic supplements over a set period. Post-intervention results showed notable changes in gut microbiota in both groups, with improvements in microbial diversity and balance. Although the nature of microbial shifts varied between the ADHD and ASD cohorts, the general trend indicated a positive modulation of gut health. These microbiome changes were accompanied by reports of behavioural improvements, though responses differed between the two groups.
The findings suggest that children with ADHD and ASD not only have distinct gut microbiota compositions but also respond differently to probiotic intervention. This supports the potential of probiotics as a supplementary therapy for neurodevelopmental conditions, while also emphasizing the need for personalized approaches due to the varied microbiome dynamics and behavioural outcomes. Further research is needed to better understand the mechanisms linking gut microbiota to brain function and behaviour in these populations.
Alpha diversity indexes for the ADHD and ASD groups at baseline. Box plots were generated for alpha diversity indices (Chao1, Fisher’s alpha, Inverse Simpson and Shannon). No significant differences were observed. |