Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Probiotics for Stressed Students: Calming Your Gut When Exams Approach
Feeling frazzled before exams is perfectly normal. But did you know your gut might be getting stressed too? A brand‑new clinical trial looked into whether a probiotic could help buffer gut disruptions during academic pressure.
In this study, healthy students were randomly divided into two groups: one consumed a fermented milk drink containing Lacticaseibacillus rhamnosus CNCM I‑3690, while the other had a similar acidified but non‑fermented milk product. They each took two servings daily for four weeks leading up to final exams.
Using advanced microbiome profiling, the researchers tracked how the gut community changed. Interestingly, students taking the probiotic showed smaller shifts in both alpha‑diversity (overall richness in their gut microbiome) and beta‑diversity (how their microbiome changed over time) compared to the control group. In other words, their gut ecosystem was more stable under stress.
Digging deeper, the probiotic group maintained healthy levels of Ruminococcus bicirculans—a species sensitive to stress—and saw boosts in Faecalibacterium prausnitzii, a bacterium often linked to anti‑anxiety effects. Students with higher levels of F. prausnitzii reported feeling less anxious before the exams.
Functional changes in gut genes were modest—but the take‑home message is promising. A simple probiotic may help your gut stay resilient when your mind is under pressure. The team notes more work is needed, but the study paves the way for gut‑focused tools to help with mental stress and exam jitters in the future.
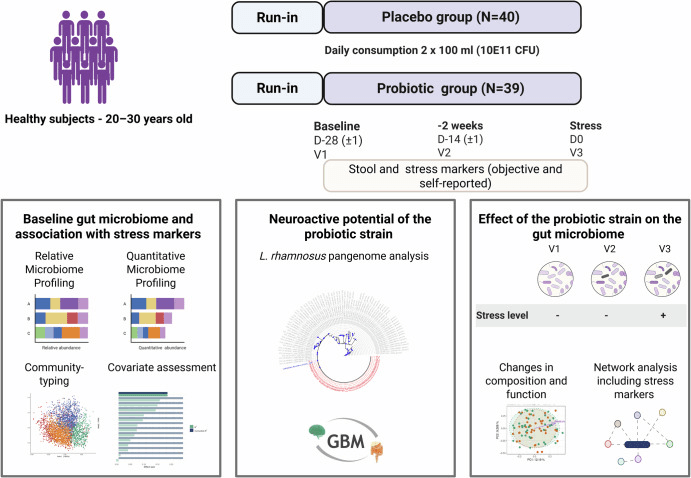
Study design and analysis performed
Gut Microbiome Clues in Melanoma: Can Your Gut Help Predict Cancer Outcomes?
Imagine if your gut bacteria could signal how well your body will respond to cancer treatment. That’s exactly what researchers investigated in a promising new study on high-risk stage III melanoma.
In the NeoACTIVATE trial, patients received a powerful mix of treatments—either a triple combo (vemurafenib, cobimetinib, and atezolizumab) if their melanoma had a BRAF mutation, or a duo of cobimetinib plus atezolizumab if it didn’t. These were given before surgery, followed by additional immunotherapy afterward.
Here’s the gut-related twist: Scientists analyzed patients’ gut microbiomes and found striking patterns. Patients with low microbial arginine biosynthesis—a function important for activating immune T cells—were much more likely to experience early distant relapse (p = 0.0005). That’s huge, because it suggests that the metabolites your gut bacteria produce might affect how well your immune system fights off residual cancer after treatment.
They also noted specific differences in which bacterial groups were present (taxonomy) and how diverse the gut ecosystem was—these linked strongly with better long‑term outcomes.
What does this mean in everyday terms? Think of your gut microbes as collaborators in cancer treatment. Their ability to produce key nutrients might help your T‑cells stay active and guard you against relapse. In the future, analyzing microbes could help doctors tailor treatments—even before surgery begins.
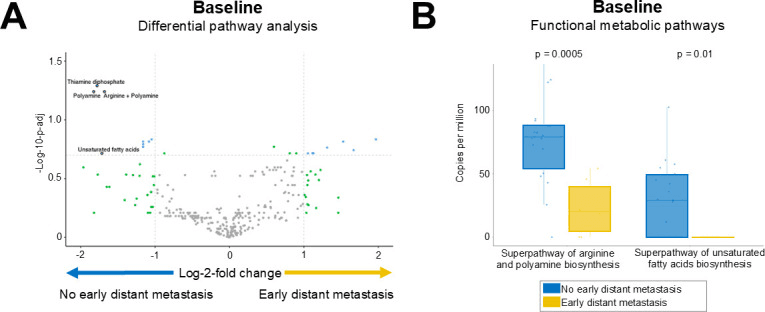
Gut microbiome functional pathways in association with early distant metastasis. (A) Volcano plot illustrates the most significantly differentially abundant pathways based on MaAsLin2. (B) Boxplots detail the relative expression of two key metabolic pathways with highly significant differences between patient groups.
Beyond Prevention: How Birth Control Shapes Vaginal Immunity and Bacteria
Your vaginal microbiome is a dynamic ecosystem, constantly influenced by hormones, lifestyle, and even your method of contraception. A recent study explored how three widely used contraceptive methods—the copper IUD, the levonorgestrel (LNG) implant, and the injectable DMPA—affect this delicate microbial environment.
The researchers followed 218 women over a year, tracking their vaginal microbiota and immune markers. They found that the copper IUD was linked to a higher prevalence of non-Lactobacillus-dominated microbiomes, which are often considered less protective. Women using copper IUDs also showed more microbial instability and higher levels of inflammation-related molecules like cytokines and antimicrobial peptides. This suggests that the copper IUD may disrupt the vaginal environment more than other methods.
In contrast, the LNG implant had a stabilizing effect. Users of this hormonal method tended to maintain a Lactobacillus-rich microbiome—particularly L. crispatus, a species known for its protective properties. Their microbial profiles were more stable over time and showed fewer signs of inflammation, pointing to a healthier and more resilient vaginal ecosystem.
The DMPA injection, interestingly, had minimal overall impact. Women using DMPA showed neither the disruptions seen with the copper IUD nor the microbiome-friendly boost seen with the LNG implant. Their microbial and immune profiles remained largely unchanged.
These findings underscore that contraceptive choices can have ripple effects beyond fertility. While all three methods are effective at preventing pregnancy, their influence on microbial and immune health varies. This research adds an important layer of understanding for those weighing their contraceptive options.
How Meditation and a Plant-Based Diet Reshape Your Gut in Just 9 Days
A recent study revealed that just nine days of meditation combined with a vegetarian diet can significantly shift your gut and oral microbiome—for the better. Researchers followed 24 people during an Arhatic Yoga retreat, where participants practiced deep meditation daily and consumed a clean, plant-based diet. The changes in their gut and mouth bacteria were measured using saliva and stool samples at the beginning, middle, and end of the retreat.
The results were surprising. By Day 3, participants' gut microbiomes had already begun to shift. Beneficial bacterial groups, including Bifidobacterium adolescentis, Bifidobacterium longum, Bifidobacterium bifidum, Lactobacillus mucosae, Lactobacillus salivarius, and Faecalibacterium prausnitzii, increased in abundance. These microbes are known to support gut health, reduce inflammation, and even improve mood through the gut-brain axis.
Interestingly, the oral microbiome also changed, with noticeable increases in microbial diversity. This suggests a broader impact of meditation and diet on the body’s entire microbial ecosystem—not just in the gut.
Whether participants were new to meditation or long-time practitioners, the microbiome benefits were consistent. The researchers believe this powerful combination of mental focus and dietary change may help foster the growth of "next-generation probiotics"—microbes that not only aid digestion but also support immunity and mental clarity.
In just over a week, mindful living and plant-based eating reshaped the microbiome in ways that could have long-term health benefits. It's a small glimpse into how lifestyle changes can ripple down to our tiniest inhabitants.
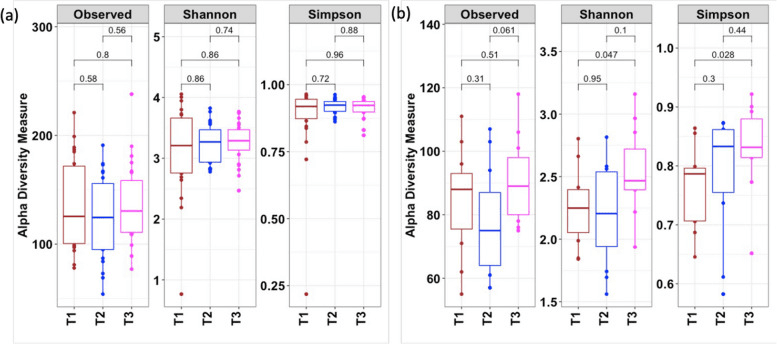
Alpha diversity measures of the fecal and oral microbiome. Timepoint variations in the alpha diversity measures of (a) fecal and (b) saliva samples. Pairwise-Wilcoxon test was performed to assess the significant difference in the alpha diversity parameters over time.
How Tamoxifen Treatment Quietly Shifts Your Gut Ecosystem
A new study explores how tamoxifen—a cornerstone therapy for postmenopausal ER+/HER2− breast cancer—affects the gut's microbial community. Researchers followed 62 women, analyzing their fecal and blood samples before and again 6–12 weeks into treatment, using 16S rRNA sequencing and precise measurement of the active metabolite endoxifen.
Interestingly, tamoxifen therapy led to a notable increase in microbial richness—a measure of species variety in the gut (p = 0.019)—suggesting the drug might gently diversify the microbiome. While the overall microbial community structure remained stable, certain bacterial groups shifted significantly: Blautia and Streptococcus increased in relative abundance, whereas a member of the Prevotella_9 group decreased.
The investigators also looked for possible links between these microbial changes and endoxifen levels in the blood—but found none that held up after rigorous statistical corrections. That means, for now, tamoxifen appears to influence the gut microbiome—but those changes don't seem to directly affect how much of its active form circulates in the body.
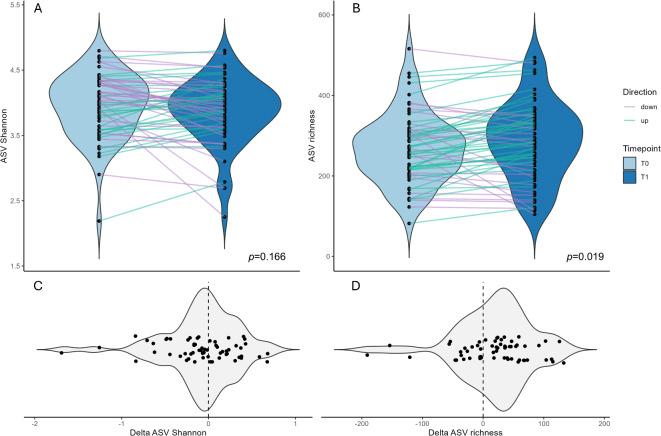
(A, B) Violin plots illustrating the distribution of alpha-diversity measurements at two different time points (T0 and T1), highlighting whether alpha-diversity increases (green line) or decreases (purple line) between paired samples. Each plot shows the density of values with individual data points overlaid as black dots. (A) Shannon index. (B) ASV richness. (C, D) Violin plots illustrating the distribution of the shifts in alpha-diversity measurements (C Δ Shannon; D Δ ASV-richness) between T0 and T1 for paired samples. The dashed line in the plot indicates zero (no change), serving as a reference point to easily identify increases or decreases in alpha-diversity.
