Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Microbial Mediators of the Mind: The Gut-Brain Axis in Alcohol-Induced Neuroplasticity
The study delves into how alcohol use disorder (AUD) affects brain health not only through direct neurochemical disruption but also by reshaping the gut microbiome, which in turn modulates brain gene expression. By analyzing data from individuals with AUD, the authors uncovered a strong correlation between altered gut bacterial composition and the expression of genes related to neuroplasticity. The microbiome-derived metabolites, including short-chain fatty acids and other signaling molecules, appear to act as intermediaries between gut dysbiosis and changes in brain structure and function, particularly in areas like the frontal cortex which are involved in cognition and behavioral control.
A key finding of the research is that patients with AUD exhibit significant microbial dysbiosis—characterized by reduced microbial diversity and increased abundance of pro-inflammatory species—which coincides with a downregulation of neuroplasticity genes such as BDNF and ARC. This suggests that the gut microbiome is not merely a bystander but a critical player in the neurobiological consequences of chronic alcohol exposure. The increased gut permeability and systemic inflammation stemming from dysbiosis are proposed mechanisms linking microbial imbalance to impaired brain plasticity and function.
These insights open new avenues for therapeutic intervention. Restoring a healthy microbiome using probiotics, prebiotics, or even fecal microbiota transplantation could help reverse some of the brain alterations observed in AUD by modulating inflammation and promoting neurogenesis. The study not only adds depth to the gut-brain axis literature but also provides a rationale for integrating microbiome-focused strategies into the treatment landscape for alcohol-related neuropsychiatric disorders.
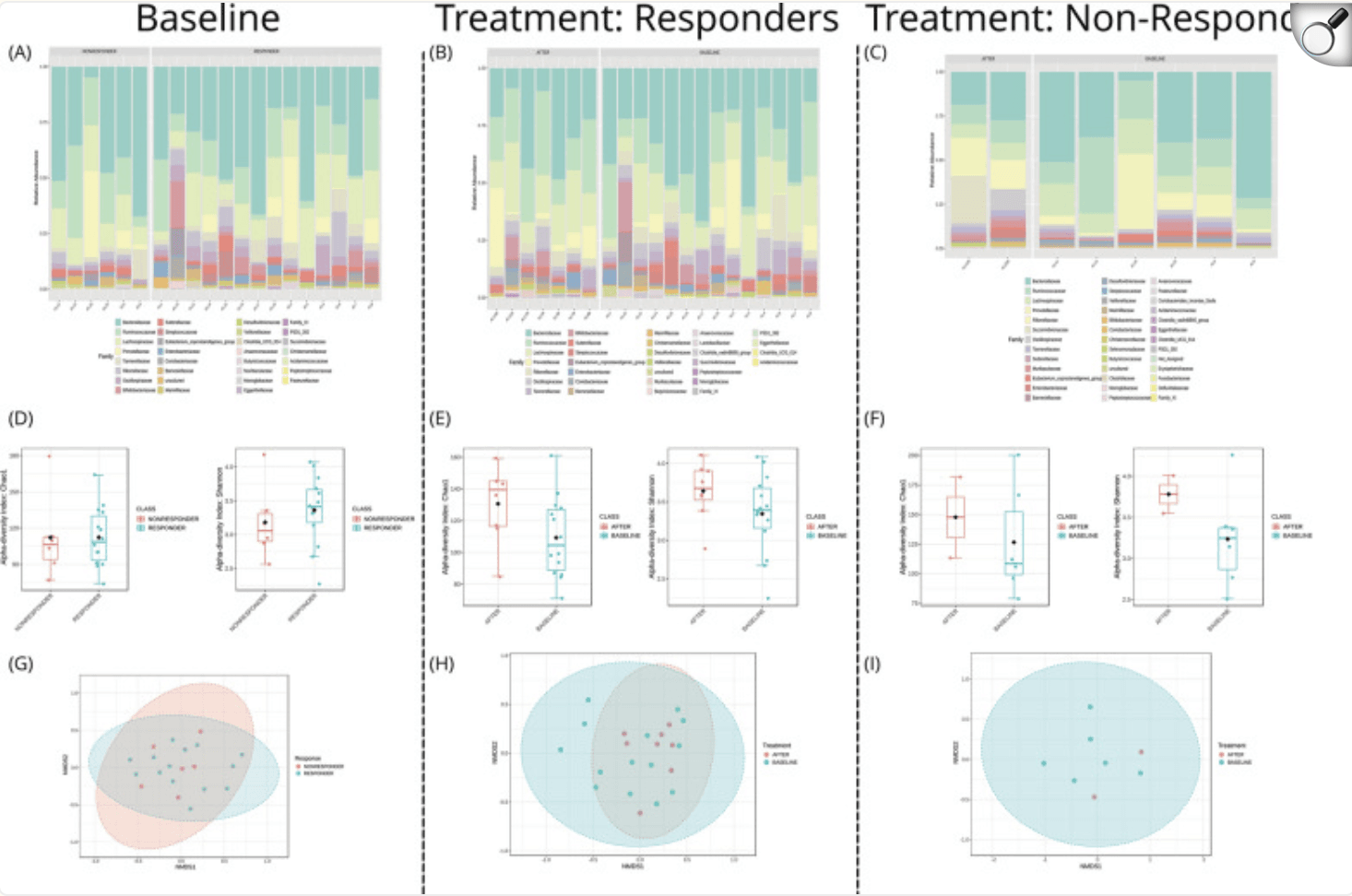
Microbiome analysis at baseline and post-intervention of responders and non-responders. The analyses cover (A)–(C) relative abundance of microbial families (D)–(F) alpha-diversity with Chao1 and Shannon indices (G)–(I) beta-diversity NMDS plots.
Probiotic Support in Neonatal Jaundice: A Gut Microbiome Perspective
Neonatal jaundice, affecting nearly 60–70% of infants in Asia, is typically treated with phototherapy to reduce elevated bilirubin levels. However, this treatment is not without drawbacks—disruption of the gut microbiome being a key concern. In a recent randomized, double-blind, placebo-controlled clinical trial involving 300 full-term neonates, researchers evaluated whether supplementing phototherapy with specific probiotics could enhance bilirubin clearance while supporting microbial health. Two strains were tested: Lactobacillus salivarius AP‑32 and Bifidobacterium animalis subsp. lactis CP‑9, both administered alongside standard phototherapy. The primary outcome was bilirubin reduction, but the study also closely examined gut microbiome changes.
Both probiotic groups demonstrated significantly improved outcomes compared to the placebo. Not only was there a faster reduction in bilirubin levels, but infants receiving L. salivarius also experienced shorter hospital stays. Microbiome analysis showed increased alpha-diversity in both probiotic groups, suggesting a richer and more resilient gut ecosystem. Importantly, the supplemented strains successfully colonized the gut in both probiotic groups. These changes are not just compositional; they imply functional benefits such as improved gut barrier integrity and reduced activity of β-glucuronidase, an enzyme that contributes to bilirubin reabsorption in the intestine.
This study highlights a compelling interaction between the gut microbiome and bilirubin metabolism, suggesting that probiotics can enhance phototherapy efficacy by promoting a healthier intestinal environment. The findings provide strong evidence for incorporating targeted microbial support in neonatal jaundice management. They also pave the way for precision probiotic therapies, as the differential effects of Lactobacillus and Bifidobacterium strains underscore the importance of selecting the right microbes for clinical outcomes.
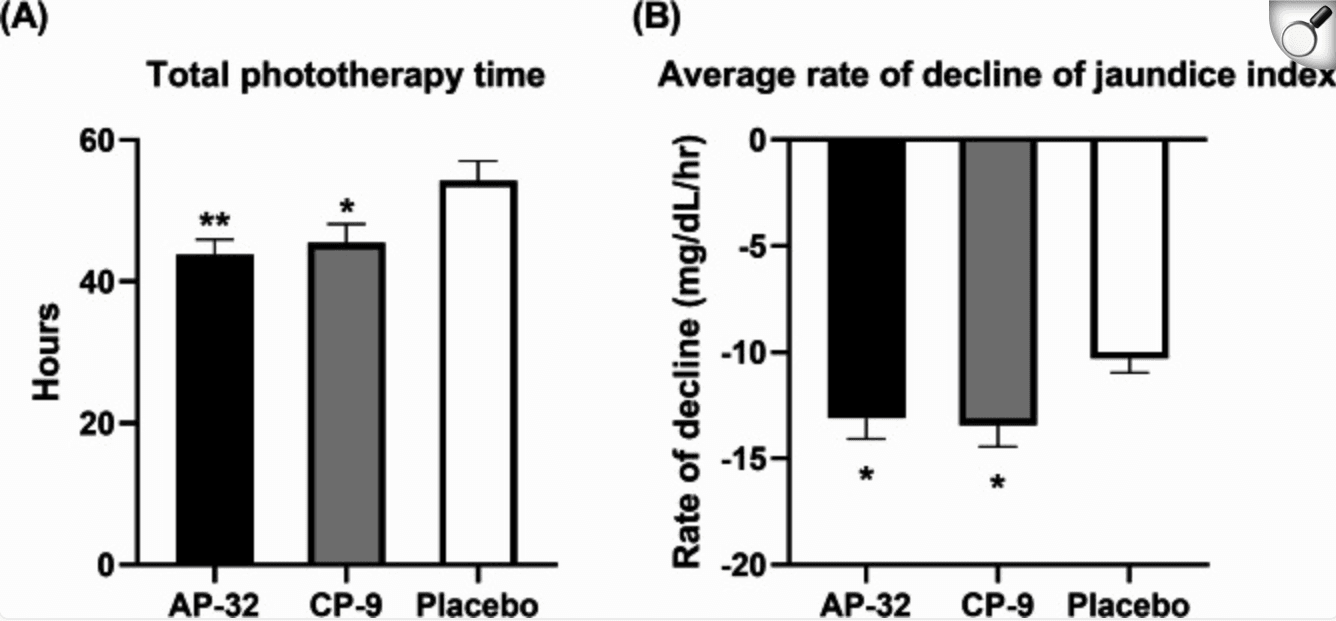
During the trial period, both (A) the total duration of phototherapy and (B) the bilirubin declined rate were evaluated. Jaundice index declined rate = (jaundice level at discharge − jaundice level at admission) / duration of phototherapy. Significance levels were indicated as follows:*P < 0.05 and **P < 0.01 compared to placebo.
Microbiome Modulation with Prebiotics in Autism: Insights from a Clinical Trial
A double-blind randomized controlled trial investigated the impact of prebiotic supplementation on gut microbiota compositions in children with autism spectrum disorder (ASD), alongside behavioral and gastrointestinal outcomes. Participants received prebiotics over an 8‑week period, with serial sampling for microbial community profiling via 16S rRNA gene sequencing. The core finding was a statistically significant modulation of the gut microbiome: children receiving prebiotics showed increased relative abundance of Bifidobacterium and reduced levels of potentially pro-inflammatory Clostridium species. These shifts mirrored a community state more often associated with neurotypical gut ecosystems.
Alpha-diversity metrics remained stable across groups, indicating that prebiotic intervention did not disrupt overall microbial richness. However, beta-diversity analyses revealed a distinct clustering of post-supplementation microbiomes in the treatment arm compared to baseline and controls, suggesting compositional restructuring. Functionally, inferred metagenomic pathways pointed to enhanced short-chain fatty acid (SCFA) synthesis capacity—particularly butyrate and propionate—which are known modulators of gut–brain signaling and intestinal barrier integrity.
Correlations between microbial taxa shifts and clinical measures were observed. The increase in Bifidobacterium correlated inversely with the severity of gastrointestinal symptoms and, to a lesser extent, certain behavioral outcomes. Though behavioral improvements were modest, the microbiome modulation was consistent with hypothesized mechanisms linking the gut ecosystem to neurological and physiological function. This trial supports the concept that targeted modulation of pediatric ASD gut microbiota via prebiotics is feasible, safe, and biologically impactful, warranting larger studies to dissect causality and longitudinal effects.
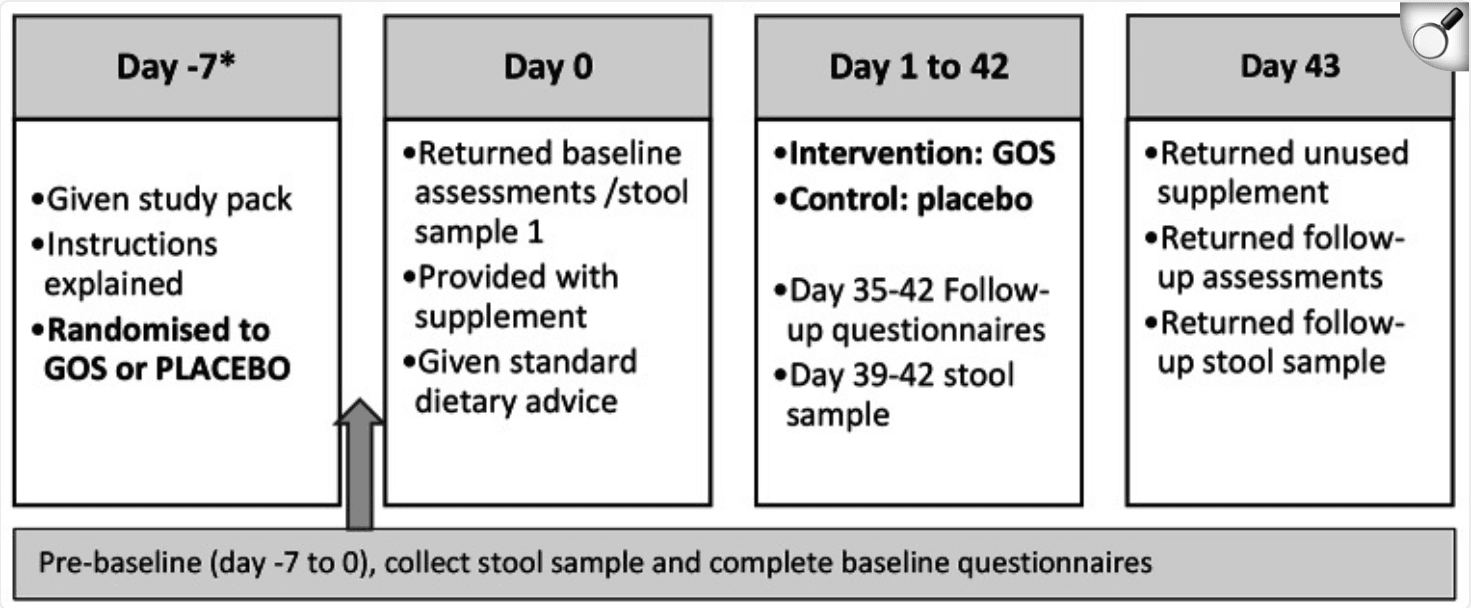
Intervention Time Line
Gut-Level Gains: The Microbiome Impact of Balanced Energy-Protein Supplementation in Pregnancy
Maternal nutrition plays a pivotal role not only in fetal development but also in shaping the microbiome landscape of both mother and child. In this randomized controlled trial conducted in Burkina Faso, the effects of balanced energy-protein (BEP) supplementation during pregnancy and lactation were explored with a focus on the gut microbiome. Using deep shotgun metagenomic sequencing, researchers examined stool samples from 152 mother–infant pairs at multiple time points. The findings reveal that BEP supplementation significantly increased maternal microbial diversity and gene richness, especially in the postpartum period. These changes suggest a more robust and functionally versatile microbiota, characterized by enhanced network connectivity during pregnancy and increased modularity after childbirth, pointing to a dynamic microbial restructuring across maternal life stages.
Notably, the supplementation led to an enrichment of Bacteroides fragilis and a reduction in pro-inflammatory and energy-intensive pathways such as lipopolysaccharide biosynthesis and carbohydrate uptake systems. These changes reflect a shift in microbial priorities, potentially reallocating energy to support fetal growth. In infants, those born to BEP-supplemented mothers showed accelerated microbiome maturation with enhanced genes for lactose and starch metabolism, indicating an improved capacity for energy extraction from breast milk. While overall diversity was not directly linked to growth outcomes, specific taxa like Bifidobacterium breve, Streptococcus spp., and butyrate-producing Oscillospirales appeared to mediate positive effects on weight and length at six months.
This study highlights the microbiome as a critical intermediary in translating maternal nutritional interventions into infant health benefits. By reshaping maternal and infant microbial ecosystems, BEP supplementation creates a more favorable metabolic environment that supports early-life development. These findings open new avenues for maternal and infant health strategies that consider not just nutrient intake, but also the microbial pathways that process and mediate those nutrients.
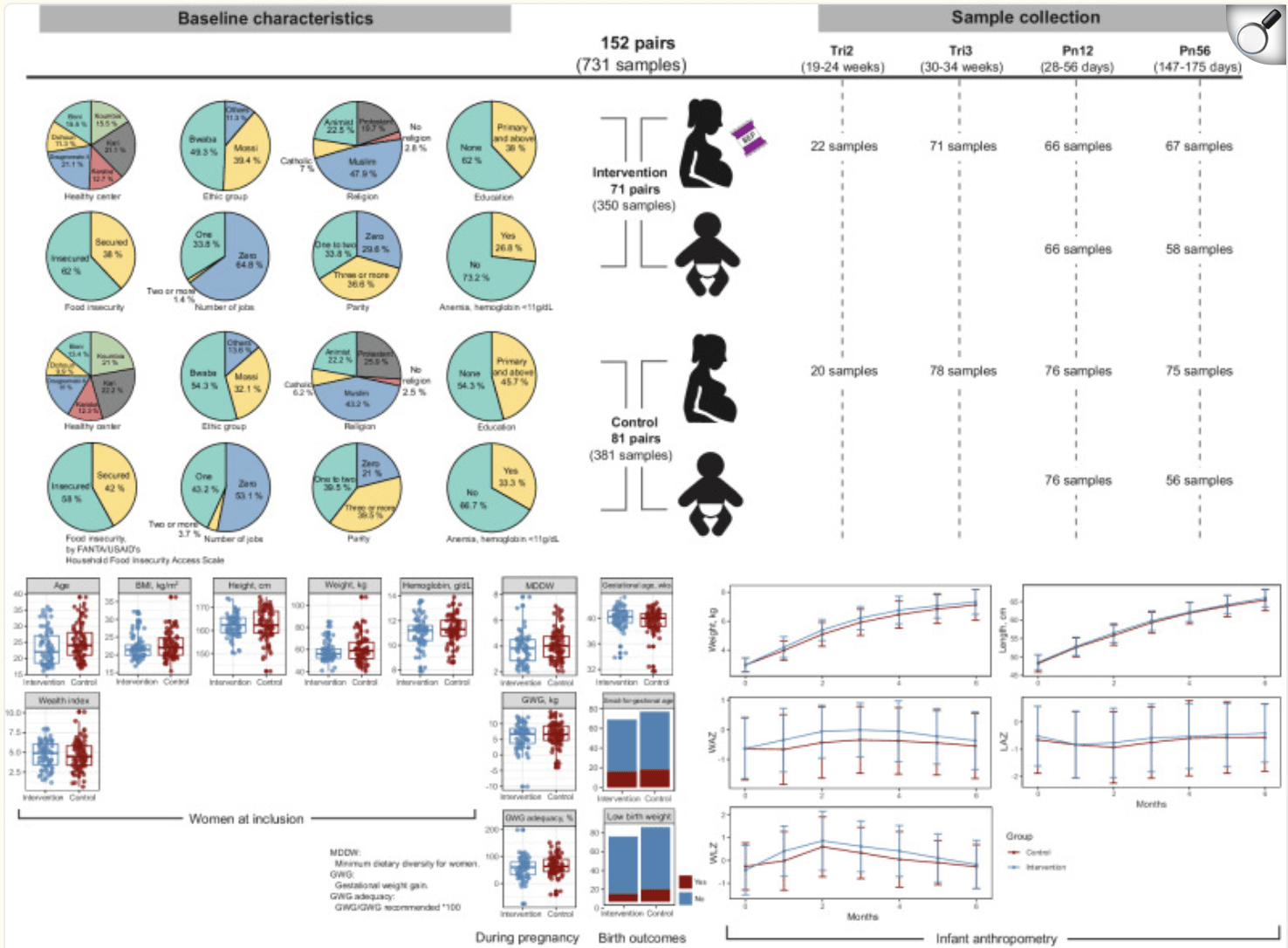
Pie charts depict the baseline characteristics of the study participants, including health center, ethnicity, religion, education level, food insecurity, number of jobs, parity, and anemia for each group. Box plots present other measures, such as the age of the participants, BMI, height, weight, hemoglobin levels, and wealth index, along with minimum dietary diversity score and gestational weight gain during pregnancy. Birth outcomes are displayed using box plots and bar plots. Infant anthropometry data are presented as growth curves for weight, length, and corresponding Z-scores (WAZ, LAZ, and WLZ) during the first six months of life, comparing the intervention and control groups. Box plots indicate median (middle line), 25th, 75th percentile (box), and the smallest and largest values within 1.5 × interquartile range (IQR) from the box (whiskers). Data points beyond this range are considered outliers (single points). Data in line plots are presented as mean ± standard error. BMI body mass index, GWG gestational weight gain, MDDW minimum dietary diversity for women, Pn12 postnatal 1–2 months, Pn56 postnatal 5–6 months, Tri2 trimester 2, Tri3 trimester 3, LAZ length-for-age Z-score, WAZ weight-for-age Z-score, WLZ weight-for-length Z-score.
Needles and Neurons: How Acupuncture Shapes the Gut Microbiome to Boost Cognition
A randomized controlled trial investigated how acupuncture influences cognitive function in individuals with amnestic mild cognitive impairment (aMCI) via the microbiota–gut–brain (MGB) axis. Over 12 weeks, patients receiving acupuncture showed a clinically meaningful improvement in cognition (ADAS-Cog scores decreased by –3.94 points) compared to a control waitlist group (+1.72 points; net difference of –5.66).
The researchers employed a multi-omics approach—metagenomics of gut microbiota, brain imaging, and serum metabolomics—to uncover underlying mechanisms. Acupuncture modulated brain regional homogeneity in areas such as the left medial orbital frontal cortex and cingulate cortex. Crucially, it also altered gut microbial composition: notably increasing the relative abundance of Ruminococcus sp. AF43_11 and Eubacterium coprostanoligenes. Both are recognized for producing beneficial short-chain fatty acids and supporting gut barrier integrity.
These microbial shifts were accompanied by changes in circulating metabolites—specifically, increased levels of certain lipid mediators and nucleic acid derivatives—that correlated with cognitive improvement. The findings suggest that acupuncture may exert its neuroprotective effects not only through direct brain modulation, but also via reshaping the gut microbial community and its metabolic outputs—highlighting a tangible MGB‑axis mechanism.
In essence, this study provides compelling clinical evidence that acupuncture enhances cognitive function in aMCI patients by influencing gut microbiota composition and metabolite profiles, which in turn relate to changes in brain activity. It underscores the therapeutic potential of microbiome modulation in neurodegenerative risk and prompts deeper exploration of gut-targeted strategies in cognitive care.
Stay tuned to unravel the latest discoveries on dynamic human-microbe interactions!