Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
When Pathogens Don’t Mean Problems: Rethinking the Microbiome Around Dental Implants
A 5-year clinical study explored how the microbial environment around dental implants evolves over time—and what that might mean for implant health. While the trial also compared early (4-week) versus conventional (8-week) loading of implants with a multiphosphonate-treated surface, one of its most intriguing findings came from the microbiome analysis. Over time, researchers noticed a significant shift in bacterial populations: microbes typically associated with gum disease, such as Porphyromonas gingivalis and Tannerella forsythia, became more dominant, while several beneficial or neutral species declined.
Surprisingly, despite this microbial shift toward a more pathogenic profile, the implants remained clinically healthy and bone levels remained stable in nearly all patients. Only one implant showed notable bone loss, and that was due to a traumatic extraction of a neighboring tooth. This disconnect between microbial changes and bone loss challenges the assumption that the presence of disease-associated bacteria automatically signals implant failure. It suggests that other factors—like host immune resilience, implant design, and tissue integration—may play a more protective role than previously thought.
These findings point to a more nuanced view of oral microbiology in implant dentistry. Rather than focusing solely on the presence of harmful microbes, future approaches may need to consider the broader balance of the microbiome and how it interacts with the body's defenses. This research underscores the importance of long-term monitoring and supports the idea that implants can remain successful even in the face of microbial changes—if supported by good clinical practices and robust host responses.
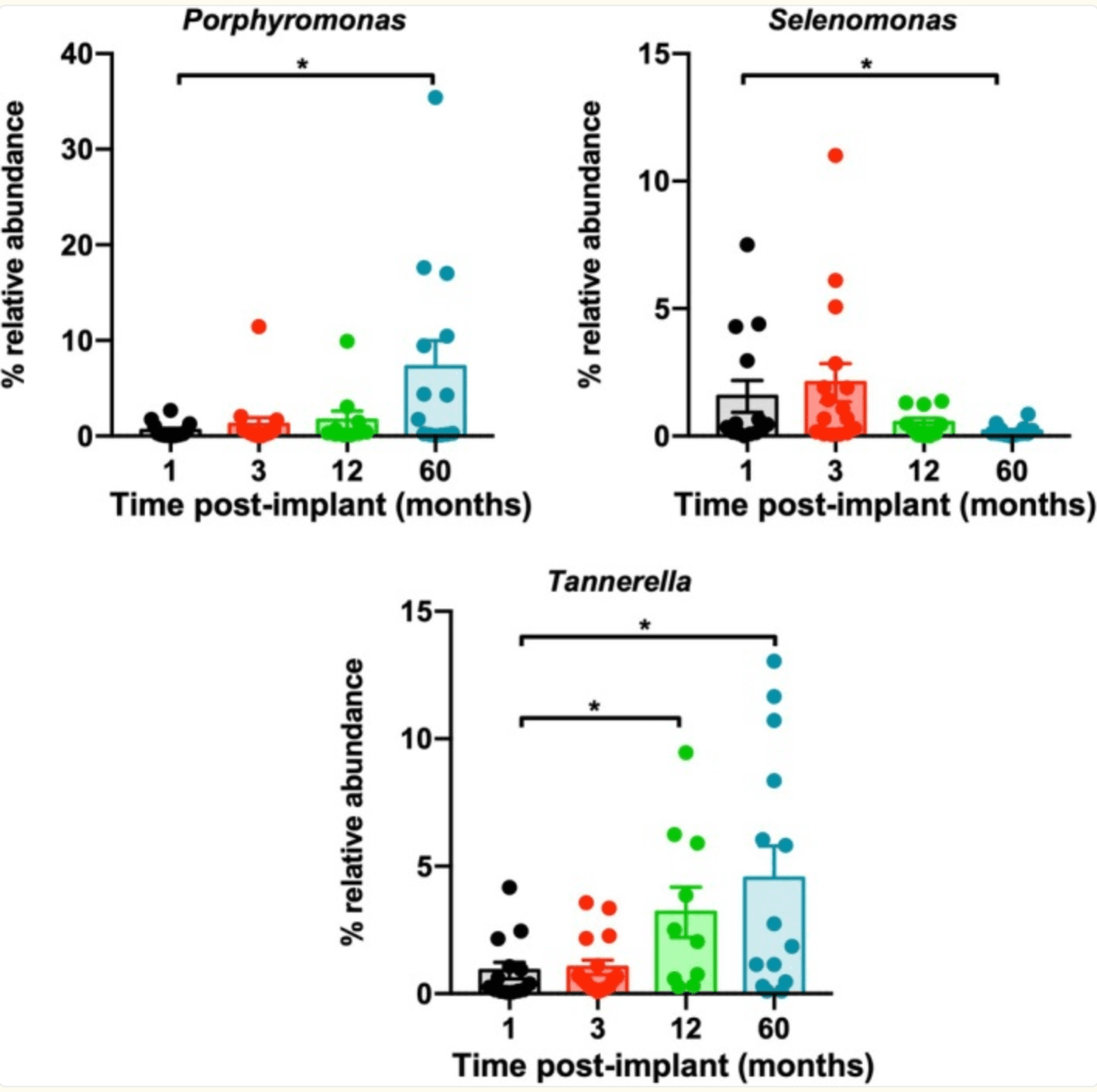
Post‐implant time evolution of oral microbiota at the genus level. Representation of the relative abundance over time of genera Porphyromonas, Tannerella, and Selenomonas. Values are expressed as mean ± SEM (n = 10–16). *p < 0.05 compared with samples collected at 1 month.
Greenspace Exposure Boosts Gut Health—One Walk at a Time
A recent randomized controlled trial from China has revealed that spending time in natural greenspaces can positively influence gut microbiota diversity. In the study, 30 healthy university students were assigned to spend two hours daily for a week in one of three environments: a vegetated park, an open but non-vegetated area, or an indoor classroom. After just one week, only the group exposed to the greenspace showed a significant increase in gut microbial diversity, measured by both species richness and phylogenetic complexity. This suggests that even short-term contact with natural environments can stimulate a measurable shift in the gut’s microbial ecosystem.
Beyond just increasing diversity, the greenspace group exhibited notable changes in the types of microbes present. Several bacterial genera associated with health benefits became more abundant, and functional predictions based on microbial composition showed reduced associations with pathways linked to substance dependence, cancer, and infectious disease risk. This implies that greenspace exposure doesn’t just diversify the microbiota—it may actively nudge it toward a more health-promoting configuration. These effects were not seen in the other two groups, highlighting the unique biological influence of living vegetation and environmental microbiota.
Interestingly, while gut microbiota responded to greenspace exposure, no changes were observed in the oral microbiota across any of the groups. This indicates that the effect of environmental exposure may be organ-specific, potentially influenced by ingestion of environmental microbes or gut-brain-stress pathways. Overall, the findings support the idea that natural environments can have rapid and beneficial effects on internal microbial communities—adding scientific weight to the age-old wisdom that spending time in nature is good for you, right down to your gut.
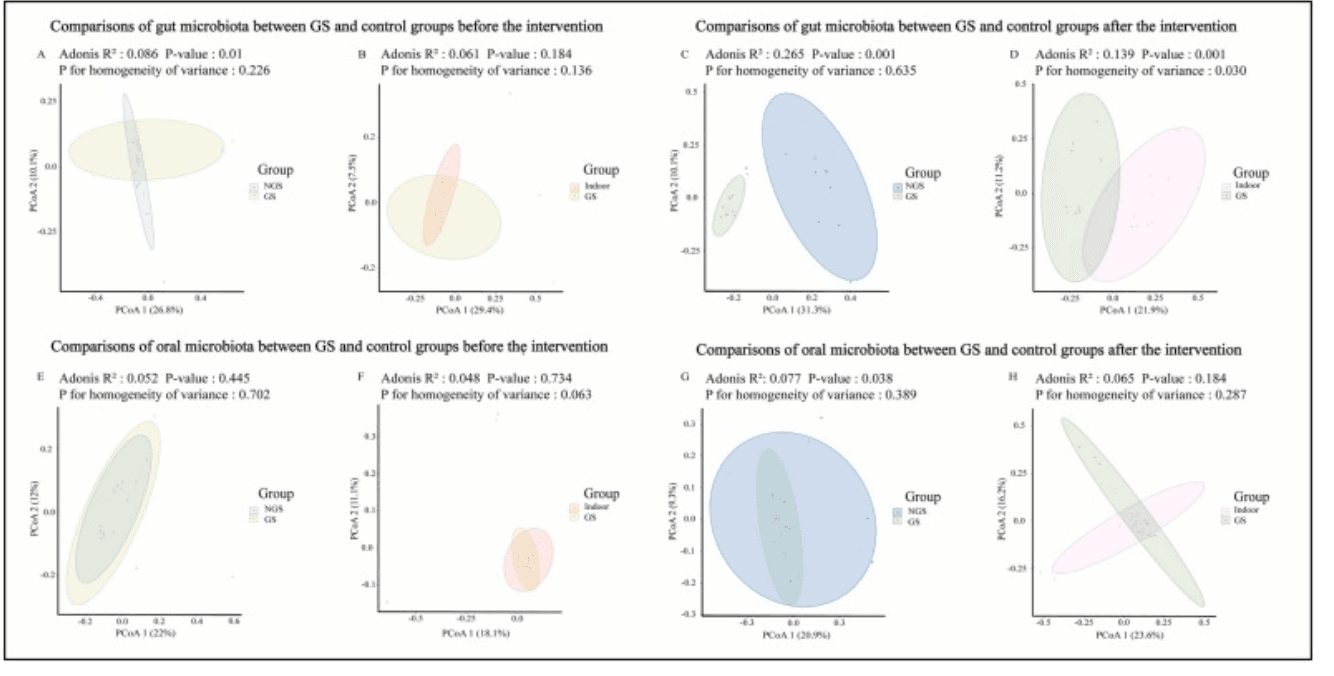
Differences of beta-diversity across groups. Comparisons of beta-diversity of human gut microbiota between GS and NGS groups as well as between GS and indoor groups before the intervention (A and B) as well as after interventions (C and D); comparisons of beta-diversity of human oral microbiota between GS and NGS groups as well as between GS and indoor groups before the intervention (E and F) as well as after interventions (G and H).
From Gut to Skin: How FMT Modulates Microbiota and Eases Eczema
In a groundbreaking randomized, double-blind, placebo-controlled clinical trial, researchers explored the potential of fecal microbiota transplantation (FMT) to treat moderate-to-severe atopic dermatitis (AD). While previous studies have hinted at a connection between gut microbiota and skin inflammation, this trial provided direct evidence that modifying the gut ecosystem through FMT could influence disease outcomes. Over 16 weeks, patients receiving weekly oral FMT capsules showed significant improvements in eczema severity, and more than 80% achieved a 50% reduction in their EASI scores by week 8—without any serious adverse effects.
The gut microbiota of FMT-treated patients underwent measurable changes. Although overall microbial diversity (alpha diversity) did not shift drastically, the composition of the microbiome (beta diversity) changed significantly after treatment. Notably, the bacterial species Megamonas funiformis increased in abundance and remained elevated throughout the treatment window. This species was strongly correlated with improvements in clinical scores and contributed to the enrichment of the 1,4-dihydroxy-6-naphthoate biosynthesis II pathway, a microbial route involved in vitamin K2 synthesis. The persistence of these changes, absent in the placebo group, underscores the impact of FMT in reshaping the gut ecosystem in a clinically meaningful way.
These microbiome shifts were accompanied by immunological modulation, including reduced Th2/Th17 cell populations and decreased serum TNF-α—markers closely tied to AD inflammation. The findings suggest that FMT not only introduces beneficial bacteria but also recalibrates immune responses via microbial metabolites like short-chain fatty acids and possibly vitamin K2. This study strengthens the case for using microbiota-based therapies in chronic inflammatory skin diseases and highlights M. funiformis as a promising biomarker or therapeutic target in the management of atopic dermatitis.
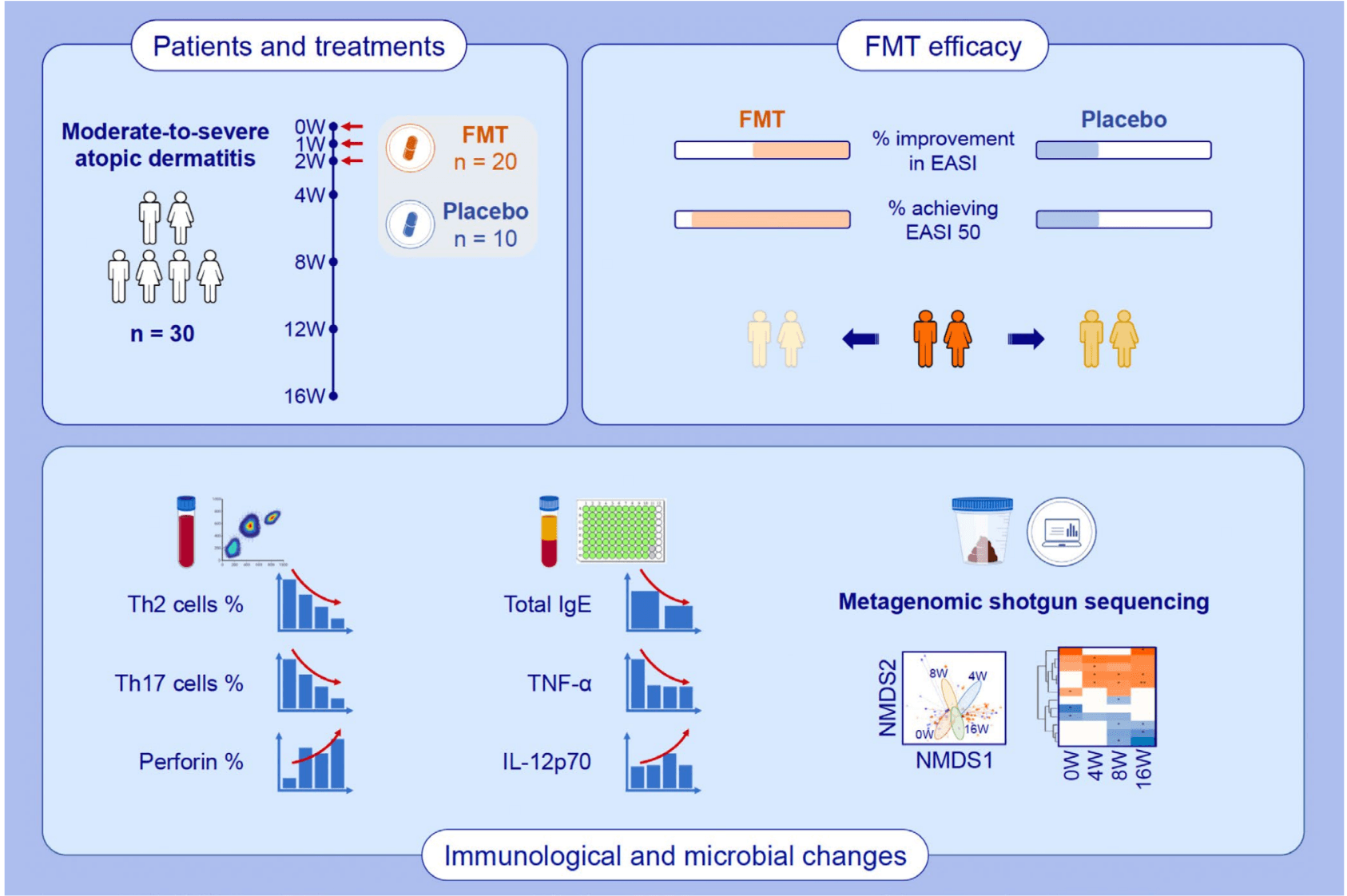
FMT showed good efficacy and safety for treating patients with moderate-to-severe AD. FMT treatment exerted immunomodulatory effects on the frequencies of inflammatory cells and the levels of cytokines associated with AD. The composition and functional pathways of gut microbiota from AD patients changed following FMT treatment. Abbreviations: AD, atopic dermatitis; EASI, eczema area and severity index; FMT, fecal microbiota transplantation; IgE, immunoglobulin E; IL, interleukin; NMDS, non-metric multidimensional scaling; Th, T helper cell; TNF-α, tumor necrosis factor alpha; W, week.
Diverging Microbial Metabolites in Mental Health: SCFAs in Schizophrenia vs. Bipolar Disorder
Emerging research continues to spotlight the gut microbiome’s role in mental health, particularly through its production of short-chain fatty acids (SCFAs)—key microbial metabolites with neuroactive and immunomodulatory functions. This study took a deep dive into the gut-derived SCFA profiles of individuals with schizophrenia (SZ) and bipolar disorder (BD), making it the first to directly compare the two. Though total SCFA levels were slightly higher in the SZ group, the core insight lies in the subtle but potentially meaningful differences in microbial metabolic output between these two psychiatric populations. Such differences may reflect distinct alterations in gut microbial communities or functions, potentially shaped by underlying pathophysiology or treatment regimens.
One of the most telling microbiome-related findings was the strong association between body mass index (BMI) and SCFA levels, especially propionate and butyrate, regardless of diagnosis. This suggests that microbial fermentation processes are tightly linked to host metabolic status. However, factors like diet, physical activity, or even psychotropic medications did not significantly influence SCFA profiles overall—pointing to the possibility that in psychiatric populations, the gut microbiome behaves differently than in the general population. Intriguingly, sex-specific interactions were noted for acetate, hinting that both microbial activity and its physiological effects may be modulated by sex in the context of mental illness.
Despite no clear link between SCFAs and clinical symptoms or cognitive measures, the findings underscore the microbiome’s evolving significance in neuropsychiatry. Altered SCFA profiles, particularly in SZ, may indicate a more pronounced disruption of the gut-brain axis, possibly involving increased intestinal permeability or dysbiosis. While fecal SCFAs provide a snapshot of microbial output, future studies including serum SCFA levels and direct microbial sequencing could offer a fuller picture. Ultimately, these microbial signals may serve not just as biomarkers but as entry points for future microbiota-targeted therapies in psychiatric care.
Psychobiotics and the Gut Barrier: A Microbiome-Based Approach in Hemodialysis Patients
In malnourished patients on hemodialysis—a group at high risk for anxiety and depression—a randomized trial evaluated the impact of combining an oral nutritional supplement with or without a probiotic blend. The probiotics included Bifidobacterium breve, B. animalis lactis, and Lactobacillus paracasei, chosen for their potential to modulate oxidative stress, gut barrier integrity, and immune signaling. Over six months, the group receiving probiotics alongside nutritional support showed notable improvements in depressive symptoms (lower HADS-D scores) and mental quality of life (higher SF‑12 MH scores), while maintaining healthy gut permeability markers such as zonulin, LPS, and LBP.
From the microbiome's standpoint, the probiotic-supplemented group experienced meaningful shifts in gut community composition. Their fecal microbiome saw a decrease in Akkermansia, a genus often linked with gut inflammation and barrier disruption, along with lower Acidaminococcus, and an increase in Barnesiella, which is thought to help ward off pathogenic bacteria. These taxonomic changes align with improved gut barrier function and reduced systemic inflammation—key factors that could underlie the observed mental health benefits.
This trial illustrates how targeted microbiome modulation, through specific probiotic strains, can influence both physiological biomarkers and psychological outcomes. While exact mechanisms remain under investigation, the data suggest that these microbial shifts helped stabilize gut permeability and attenuate inflammatory signaling, potentially enhancing patients’ mental well-being. For clinicians treating chronically ill populations, these findings offer a promising example of a psychobiotic intervention: a strategy that harnesses microbiome pathways to support both body and mind.
