Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Early-Life Gut Genes: Curating Microbial Communities for Long-Term Wellness
Early life is a make-or-break window for shaping the gut microbiome, yet we still don’t fully understand how the infant gut decides which microbes may move in. A new mouse study in Scientific Reports zeroes in on the host side of that equation, revealing a distinctive gene-expression program in the intestinal lining of young animals that actively “curates” their first microbial tenants. By spotlighting this age-specific molecular signature, the authors offer a fresh angle on why a balanced microbiota acquired early in life can ripple forward to lifelong health.
The researchers raised germ-free C57BL/6 mice to either 4 weeks (weaning age) or 10 weeks (young adult) and performed fecal-microbiota transplants (FMTs) in every age-matched and age-mismatched combination. Strikingly, it was the age of the recipient, not the donor, that dictated which bacteria ultimately colonized. Parallel microarray analysis of ileal and colonic mucosa uncovered hundreds of genes that were up- or down-regulated only in early-life gut tissue. Integrating these datasets showed that expression of early-life genes correlated positively with butyrate-producing genera such as Roseburia and the Lachnospiraceae NK4A136 group, while those same genes were negatively linked to taxa that dominate later in life.
Taken together, the findings suggest that a transient, age-tailored gene-expression “program” in the intestinal wall acts as a welcome mat for specific commensals, steering the microbiota toward a health-promoting configuration during a narrow developmental window. Disruptions—whether by antibiotics, C-section birth, or dietary shifts—could therefore derail this host-directed selection process, with downstream consequences for immunity and disease risk. Mapping these host cues opens the door to interventions that reinforce or mimic early-life mucosal signals, offering a new strategy to correct dysbiosis before it sets the trajectory for later health problems.
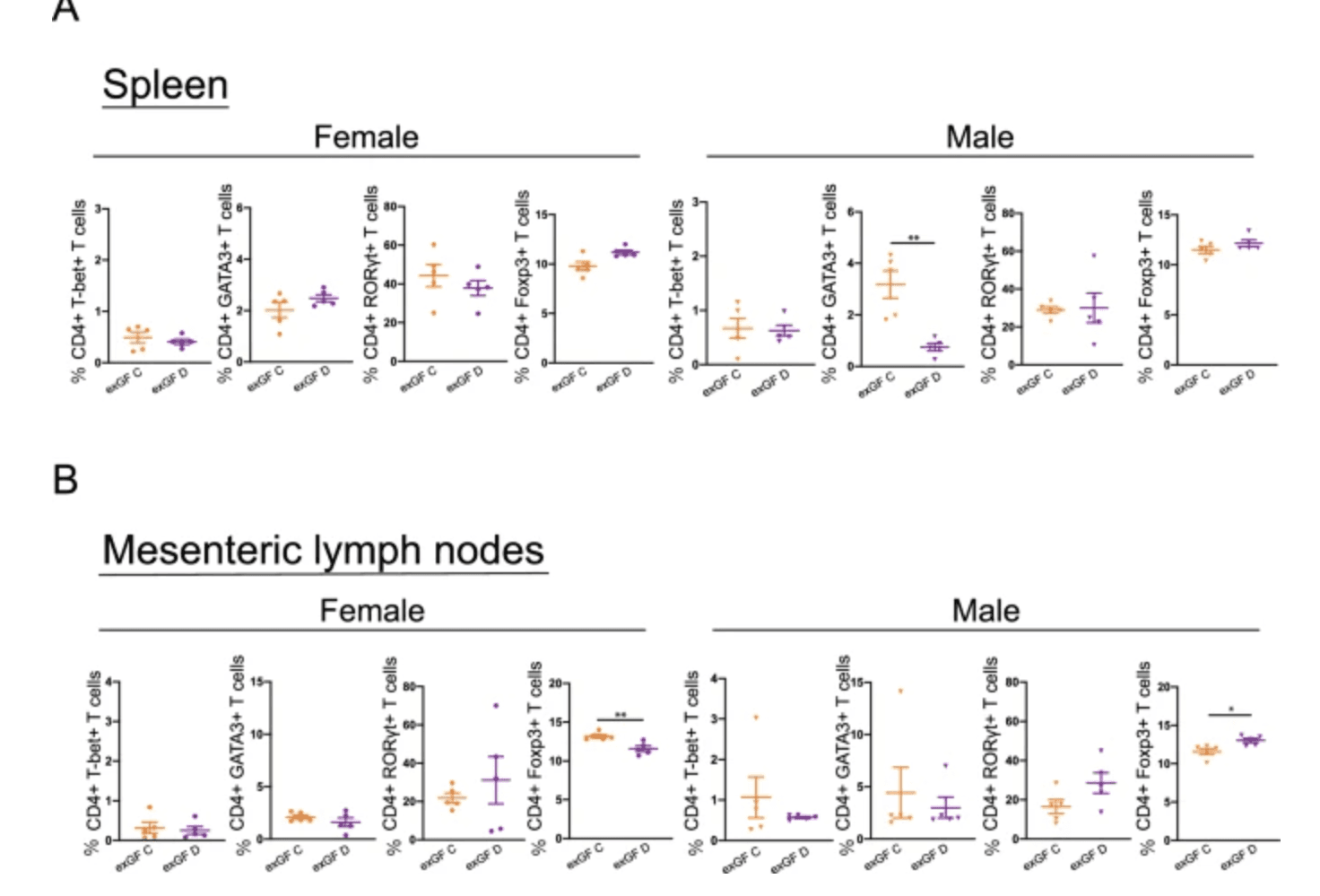
Subpopulations of CD4+ T cells in young ex-germ-free recipients of age-unmatched fecal microbiota transfer. Flow cytometric analyses of CD4+ T cells expressing T-bet+, GATA3+, RORγt+, or Foxp3+ were performed in the spleen (A) and mesenteric lymph nodes (B) of ex-germ-free (exGF) C (fecal microbiota transfer [FMT] from young donors) and exGF D (FMT from adult donors) groups. Data are described as the mean ± SEM (black filled circle: female [n = 5], black filled inverted triangle: male [n = 5]). *p < 0.05, **p < 0.01. The gating strategy for flow cytometric analyses is shown in figure. |
The Tryptophan Trail: Linking Gut Microbes to Autism Symptoms
Emerging evidence suggests that neuro-active chemicals churned out by our gut microbes may help script the brain differences seen in autism. In this cross-sectional study, researchers combined fecal metabolomics, task-based fMRI and detailed behavioural testing in 43 children with autism spectrum disorder (ASD) and 41 neurotypical peers aged 8–17. They zeroed in on the tryptophan pathway—a metabolic highway microbes use to convert dietary tryptophan into compounds that can slip across the blood–brain barrier and influence neural circuits.
The data revealed a clear biochemical signature: stool levels of kynurenate, a neuroprotective tryptophan metabolite, were significantly lower in the ASD group (Cohen’s d ≈ 0.8), even after adjusting for gastrointestinal symptoms and diet. Other indole- and kynurenine-derivatives trended lower as well. Crucially, metabolite abundances mapped onto activity patterns in classic interoceptive hubs—the mid-insula and mid-cingulate cortex—during socio-emotional and sensory tasks. In ASD participants, reduced kynurenate and related metabolites predicted stronger aberrant activation in these regions, which in turn tracked with greater autism severity, heightened disgust sensitivity and sensory hypersensitivity. Mediation analyses suggested that altered brain activity partly bridges the metabolite–symptom relationship.
Taken together, the findings paint a three-way link: shifts in gut-derived tryptophan chemistry ⇨ atypical insula-cingulate signalling ⇨ core ASD behaviours. While the design cannot prove causality, it spotlights a plausible microbial-metabolic “lever” acting on brain circuits critical for bodily awareness and emotion. If validated longitudinally, monitoring or modulating microbial tryptophan metabolism—through diet, probiotics or targeted therapeutics—could emerge as a precision-medicine strategy to ease social and sensory challenges in autism.
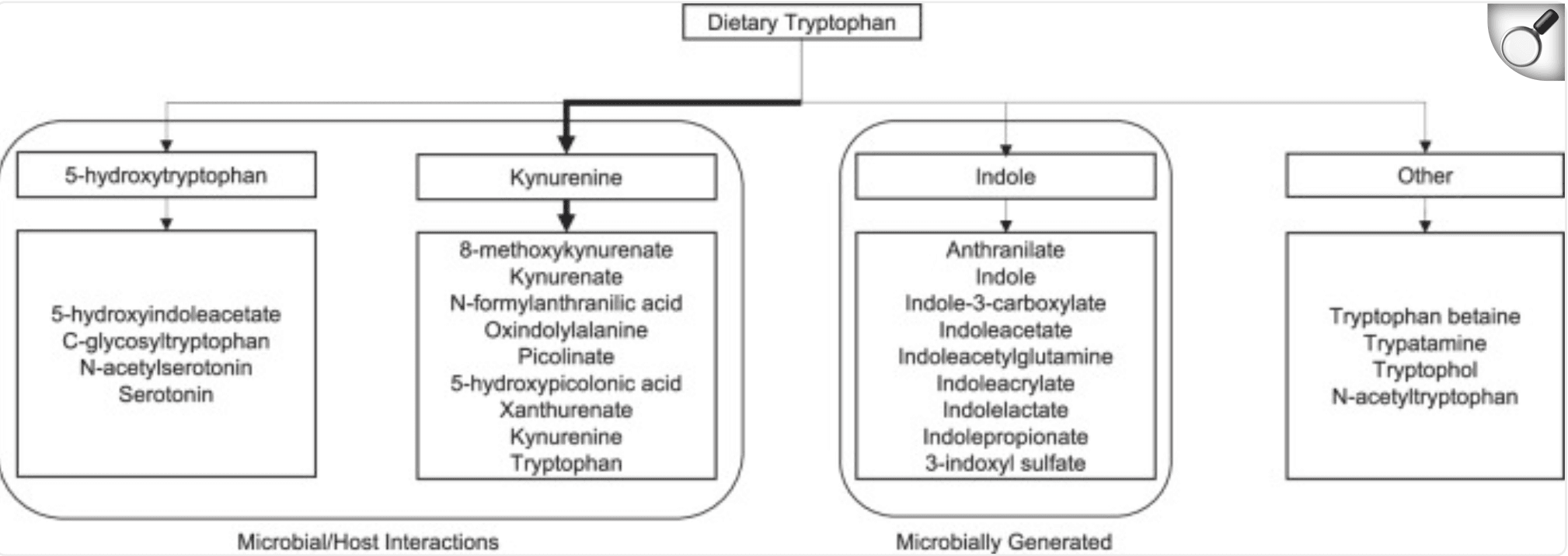
Metabolites within the tryptophan pathway. Arrow thickness represents the strength of the pathway under normal conditions. |
Time of Fat Intake May Hold the Key to Better Blood Sugar Control
A new 12-week randomized controlled trial has revealed that when we consume unsaturated fats could be just as important as what kind we consume. The study involved 70 adults with prediabetes who were assigned to one of four isocaloric diets differing in fat type—monounsaturated (MUFA) or polyunsaturated (PUFA)—and meal timing—lunch or dinner. Researchers aimed to understand not only metabolic outcomes but also changes in the gut microbiome, thanks to multi-omics profiling of stool samples.
Interestingly, the most striking improvement wasn’t linked to fat type but to timing. Participants who consumed their main fat-rich meal at lunch—regardless of MUFA or PUFA—experienced a ~50% drop in post-meal insulin levels and a ~25% increase in insulin sensitivity indices (Gutt and Stumvoll) compared to dinner-fat eaters. These metabolic benefits occurred without differences in post-meal glucose, suggesting better insulin efficiency. The lunch-fat group also showed reduced circulating saturated fatty acids, indicating improved lipid metabolism.
What tied these effects together was the gut microbiome. Lunch-timed fat consumption reshaped microbial communities in ways that boosted the transformation of bile acids—key molecules that interact with metabolic receptors like TGR5. These changes led to a higher ratio of secondary to primary bile acids, markers previously linked to better glucose control. Even more compelling, fecal transplants from lunch-fat participants improved insulin sensitivity in germ-free mice. These findings suggest that syncing fat intake with our internal clock could unlock powerful metabolic advantages, potentially offering a simple dietary tweak for managing prediabetes.
Can Probiotics Ease Constipation? New Study Says Yes—Temporarily
A new randomized, double-blind, placebo-controlled trial explored whether a five-strain probiotic supplement (Lacto-5X) could ease mild constipation in healthy adults aged 19–69. Over four weeks, 90 participants received either the probiotic (2×10⁹ CFU) or a placebo daily. By the end of the intervention, those taking Lacto-5X experienced notable improvements in stool consistency, bowel movement frequency, abdominal pain, straining, and overall satisfaction with bowel habits. However, these improvements began to fade after stopping the supplement, suggesting that continuous intake may be necessary to maintain benefits.
To understand how the probiotics worked, researchers examined the gut microbiome using metagenomics and analyzed stool metabolites. The probiotic group showed increased levels of beneficial bacteria such as Lactobacillus plantarum and Bifidobacterium, while levels of Parabacteroides—a genus linked to gastrointestinal discomfort—declined. Metabolomic analysis also revealed a rise in fecal threonic acid and a reduction in L-proline levels in the probiotic group. Notably, lower L-proline levels correlated with decreased abdominal discomfort and better stool passage, pointing to a link between microbial changes and symptom relief.
While the results are promising, the effects were short-lived and not sustained after the intervention ended. This suggests that the benefits of Lacto-5X are likely dependent on continued use. The study also did not control for dietary influences, which may have impacted results. Future research should explore long-term supplementation, dietary interactions, and whether specific strains persist in the gut to design more durable, personalized probiotic strategies for managing mild constipation.
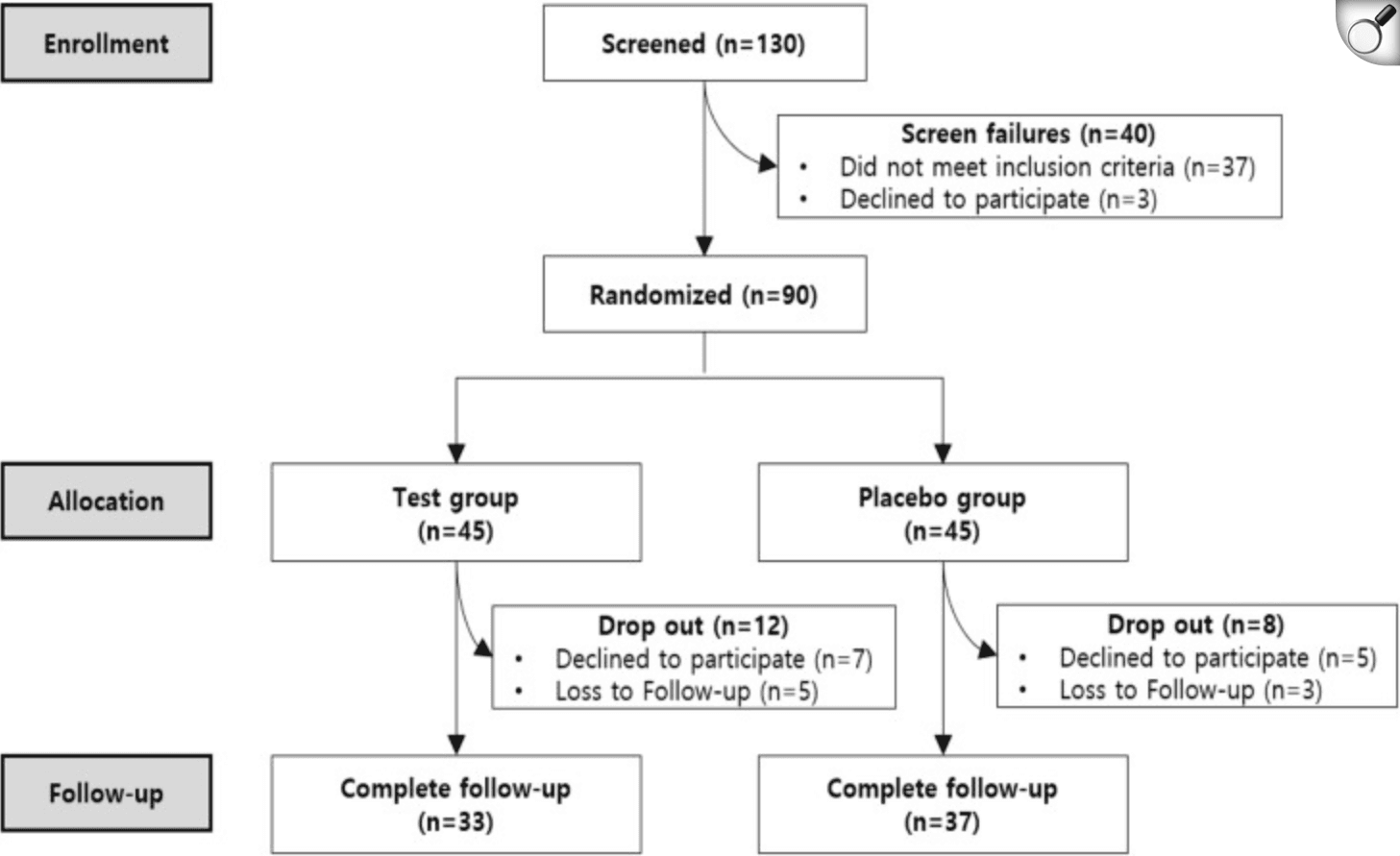
Participants flow chart
Sulforaphane and Sugar: How Gut Bacteria Influence Broccoli’s Benefits
A 12-week randomized, double-blind, placebo-controlled trial investigated whether daily supplementation with broccoli sprout extract (BSE), rich in sulforaphane, could reduce fasting blood glucose in drug-naïve adults with prediabetes. Participants received either BSE or placebo once daily, aiming for a ≥0.3 mmol/L glucose reduction. Overall, BSE yielded a modest but statistically significant decrease of 0.2 mmol/L compared to placebo (95% CI: –0.44 to –0.01; P = 0.04), though it did not meet the predefined primary endpoint. Mild gastrointestinal side effects were noted, but no serious adverse events occurred.
Pre‑specified subgroup analyses revealed that ‘responders’—those with mild obesity, lower insulin resistance, and reduced insulin secretion—experienced greater glucose reductions (~0.4 mmol/L). Intriguingly, baseline gut microbiome profiles differentiated responders from non-responders. Specifically, responders exhibited higher abundance of a Bacteroides-encoded transcriptional regulator involved in converting sulforaphane precursors into the bioactive compound. This gene operon’s prevalence correlated with increased serum sulforaphane levels, suggesting that gut microbial composition influenced treatment efficacy
These findings suggest that the metabolic impact of BSE depends on individual physiology and gut microbiota. While overall glucose-lowering effects were modest, certain individuals showed clinically meaningful responses. The study highlights the importance of accounting for microbiome variability in nutritional interventions and suggests potential to personalize sulforaphane-based therapies. Future trials should aim to confirm these exploratory subgroup findings, better control for gut microbiome differences, and explore whether microbiota-targeted strategies can enhance responsiveness in prediabetes management.
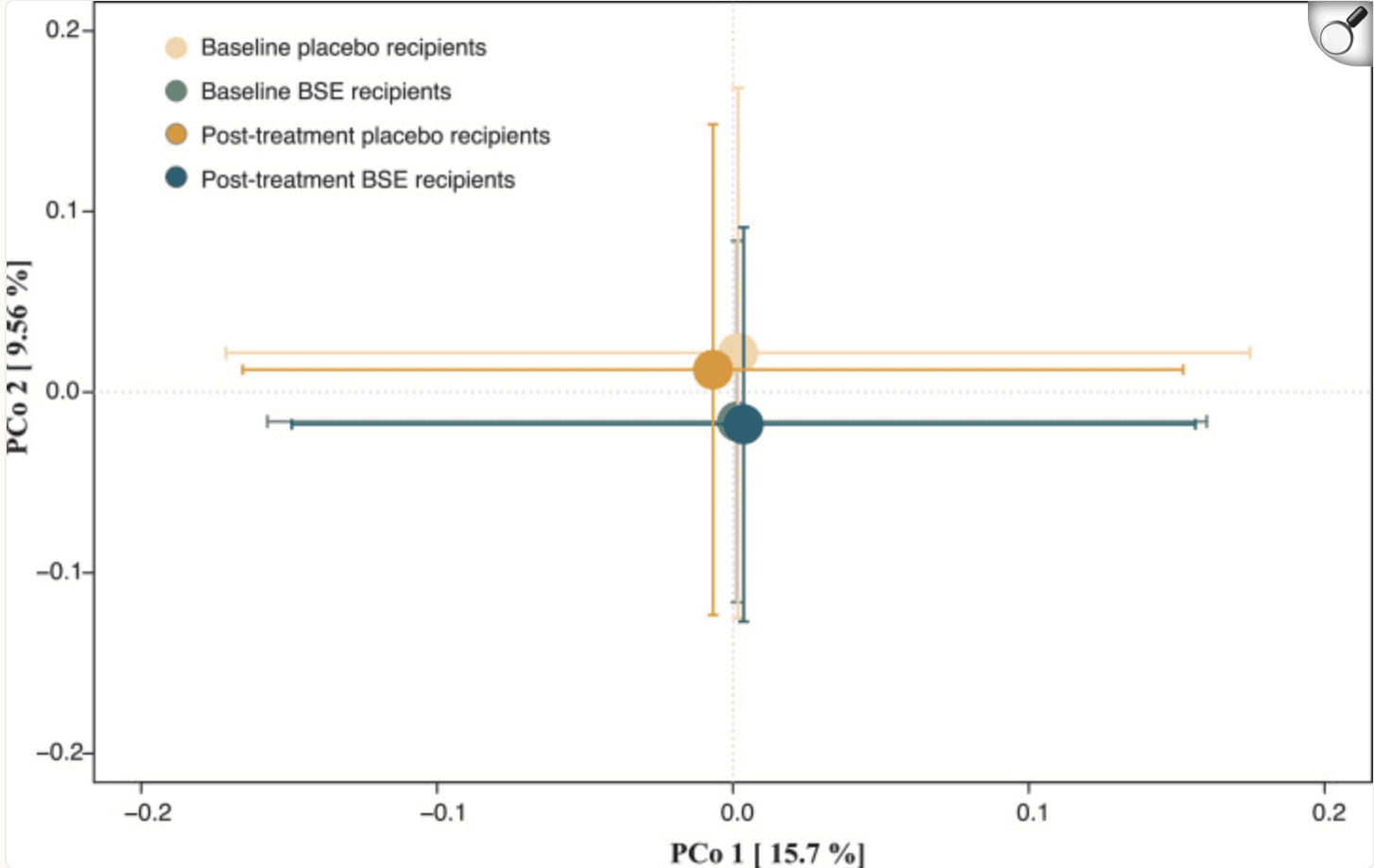
Principal coordinates analysis of species-level Bray-Curtis dissimilarities at baseline and post-treatment between study participants randomized to BSE and placebo, respectively. No significant differences were observed. |
