Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Inside the Gut–Brain Dialogue: Microbial Signals Shaping Alcohol Treatment Outcomes
The authors analyzed data from a randomized trial of zonisamide in people with alcohol use disorder to ask: can the baseline gut community and its metabolites predict who will cut back on drinking? They collected fecal samples before treatment, sequenced the 16S rRNA genes, and ran untargeted metabolomics. What emerges is a story where the pre-existing gut microbiome and its biochemical output may steer therapeutic responsiveness.
Right from the start, the authors showed that baseline community structure—how the gut bacteria are arranged—differs with sex and with whether participants were on psychotropic medication, hinting that host factors already shape the microbial canvas. More interestingly, among many bacterial genera, eleven stood out as being correlated (after adjustment) with the percent reduction in alcohol consumption over the trial. Genera like Collinsella, Eubacterium brachy group, and Marvinbryantia were positively correlated with reduction, while others such as Roseburia, Faecalibacterium, Lachnospiraceae ND3007 group, and CAG-56 tended to be negatively correlated. When participants were split into “high reducers” vs “low reducers,” their baseline gut communities clustered apart—suggesting that a signature microbiome might mark responsiveness.
Going beyond taxonomy, the metabolome sheds mechanistic intrigue. The authors found that fecal levels of GABA (γ-aminobutyric acid) at baseline correlated positively with drinking reduction; predictive functional analysis also flagged gut microbes’ GABA metabolism potential. On the flip side, 3-hydroxykynurenine, a neurotoxic intermediate in the tryptophan–kynurenine pathway, correlated negatively with drinking reduction. In comparisons between high vs low reducers, metabolic pathways involving degradation of tryptophan, histidine, glycine, and alanine showed differential representation. Importantly, the zonisamide treatment itself had minimal impact on overall microbiome diversity or major taxa shifts, suggesting the baseline state carried more weight. Though the study stops short of causality, it compellingly positions the gut microbiome and its amino-acid metabolite milieu as potential moderators or predictors of behavioral change in alcohol use disorder—and points to future possibilities for microbiome-informed personalization of addiction therapy.
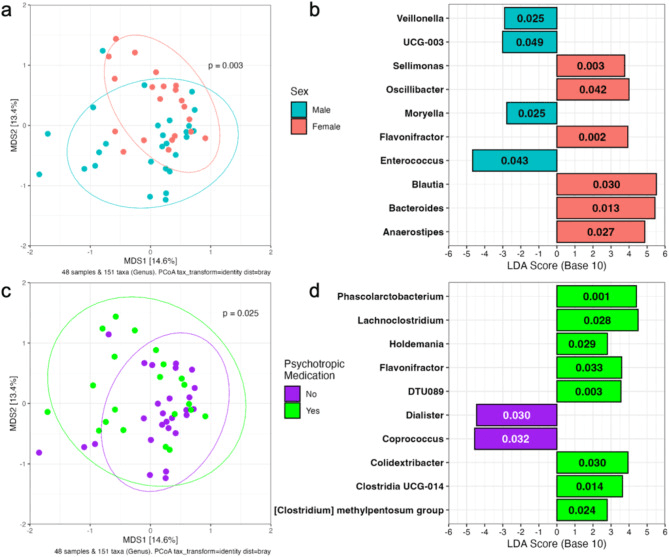
Associations between baseline gut microbiome and clinical characteristics of the participants in the study. (a) Baseline microbiome composition varies based on sex with male and female participants clustering separately in the PCoA plot determined from Bray-Curtis dissimilarity (p = 0.003, R2 = 0.051 by PERMANOVA). (b) Ten genera are overrepresented in male or female participant microbiomes. (c) Baseline microbiome composition also varies based on psychotropic medication usage controlling for sex (PERMANOVA, p = 0.025, R2 = 0.034). (d) Ten genera are overrepresented in psychotropic medication users and non-users.
From Microbes to Mood: Probiotics Awaken the Vagus Nerve
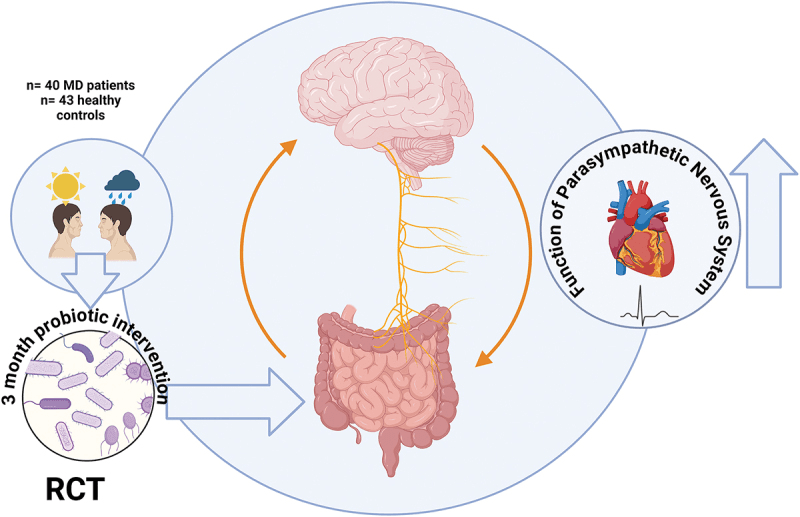
In this randomized controlled trial, scientists explored whether a multi-species probiotic could strengthen the gut–brain connection in people with major depression by enhancing vagal nerve activity, the key highway linking gut signals to the brain. The probiotic—OMNi-BiOTiC® STRESS Repair—contained nine bacterial strains, including Lactobacillus plantarum, Lactobacillus casei, Lactobacillus acidophilus, Lactococcus lactis, and Bifidobacterium bifidum are all known for anti-inflammatory and neuroactive properties. Over three months, forty-three patients with depression and an equal number of healthy controls took either the probiotic or a placebo, while their gut microbiome composition, heart-rate variability (HRV) (a marker of vagal tone), and sleep and mood parameters were monitored.
The results were striking: patients with depression who received the probiotic showed significant improvement in morning vagal nerve function, reflected by higher HRV indices like RMSSD, SDNN, and logRSA. These changes were accompanied by better sleep—shorter time to fall asleep and reduced need for sleep medication—suggesting that the probiotic may stabilize the body’s autonomic rhythms. On the microbial front, Akkermansia muciniphila, a mucin-degrading bacterium associated with gut barrier health and anti-inflammatory signaling, increased notably after three months. Members of the Christensenellales order also rose, while Ruminococcaceae declined—shifts consistent with improved gut integrity and vagal stimulation.
Interestingly, the study found that these microbial and physiological improvements emerged only after prolonged use, echoing how vagus nerve stimulation therapies for depression take months to show benefit. The findings underscore that probiotics may not act quickly but could, over time, recalibrate gut–brain communication. The authors conclude that targeted “psychobiotic” formulations—those influencing vagal signaling and microbial diversity—might one day complement antidepressant therapy, helping restore balance to both the microbiome and the mind.
Graphical Abstract
Beyond Gluten: The Microbial Story of Recovery (and Relapse) in Celiac Disease
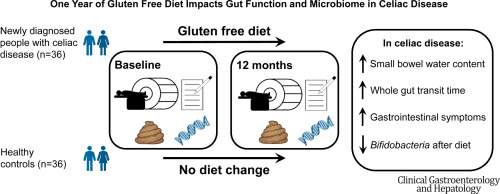
In people with celiac disease, the gut has already endured immune attack and inflammation. This study asked: after one year on a strict gluten-free diet (GFD), how much does the gut microbiome recover—or remodel itself—and what lingering changes remain? Using gut microbiome sequencing and clinical measures of gut function, the authors found a mixed picture of recovery and trade-offs under the microbial lens.
On the upside, the GFD did bring improvements in symptoms and gut transit: patients generally felt better, with modest gains in bowel movement speed. Yet when they zoomed into the microbiome, the story was more complex. Overall microbial richness and diversity remained lower than in healthy controls, and several “beneficial” bacterial groups remained depressed. Notably, Bifidobacterium — a genus often seen as friendly fiber-eaters and gut modulators — declined further on gluten withdrawal, likely because the removal of cereal fibers stripped away a key carbon source for them. Meanwhile, some bacteria associated with protein degradation—such as Escherichia and Peptostreptococcus—became more abundant, perhaps exploiting the changed nutrient landscape that now leans more on non-gluten plant sources or host-derived substrates.
These shifts carry mechanistic implications: with fewer fiber-fermenting microbes, the production of short-chain fatty acids (especially butyrate) may remain suppressed, weakening gut barrier integrity and anti-inflammatory signaling. The relative rise of proteolytic bacteria may also tip the balance toward more hydrogen sulfide, ammonia, or other metabolites that strain the mucosa. The authors suggest that a “blank slate” gluten-free diet might heal the immune insult, but it doesn’t rebuild a robust microbial ecosystem by itself. For patients still suffering persistent GI symptoms despite dietary adherence, supplementing with prebiotics (fiber that feeds beneficial microbes) or probiotics targeting Bifidobacterium and butyrate producers could help coax the microbiome back toward resilience. In other words, healing the gut in celiac disease may require both immunological peace and microbial regeneration.
Graphical Abstract
Unraveling Parkinson’s Through the Gut’s Protein Code
This study explores a fascinating and emerging link between the gut microbiome and Parkinson’s disease (PD)—but through a new lens: the gut’s own protein production machinery. Using metaproteomics, the researchers mapped how gut microbial proteins differ between patients with early Parkinson’s and healthy individuals, revealing functional disruptions that could help explain the gut-origin theory of the disease.
While many microbiome studies focus on bacterial abundance, this one dives deeper into what those microbes are doing. The researchers found that microbial proteins involved in carbohydrate metabolism, lipid processing, and amino acid synthesis were markedly altered in PD. Notably, there was an overrepresentation of proteins linked to pro-inflammatory pathways, suggesting that the gut’s microbial activity in Parkinson’s may prime the immune system toward chronic inflammation. Genera such as Akkermansia, Bacteroides, and Ruminococcus—already known to influence gut barrier function—showed distinctive protein-level shifts. Increased Akkermansia proteins aligned with mucin degradation, while reduced Bacteroides metabolic enzymes hinted at compromised SCFA (short-chain fatty acid) synthesis, particularly butyrate, a key molecule for gut and brain health.
Interestingly, host-derived proteins mirrored this microbial imbalance. Elevated markers of oxidative stress, mitochondrial dysfunction, and epithelial permeability were found in stool samples of PD patients. Together, these findings suggest a feedback loop: microbial dysbiosis alters gut metabolism, weakens barrier integrity, and amplifies inflammatory signaling—factors that may propagate neurodegenerative processes along the gut–brain axis.
By translating gut microbial functions into a molecular language, the study paints a dynamic picture of Parkinson’s disease as more than a brain disorder—it is also a metabolic and microbial condition rooted in the gut. The results underscore that future PD therapies might need to restore not just microbial balance, but microbial function itself, through precision probiotics or metabolite-based interventions.
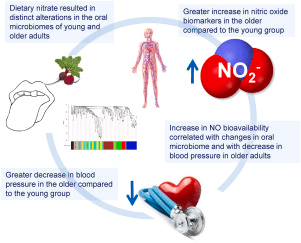
Graphical Abstract
Tiny Casualties of Cancer Treatment: How Chemotherapy Disrupts the Oral Microbiome
Chemotherapy doesn’t only target cancer cells—it also disrupts the delicate ecosystems living inside us. A recent study published reveals how cancer treatment dramatically reshapes the oral microbiome in children, offering new clues about why painful mouth sores, or mucositis, so often accompany chemotherapy.
Before treatment, the young patients’ mouths hosted a diverse balance of bacteria—common residents like Streptococcus, Neisseria, Prevotella, Veillonella, and Haemophilus coexisted in relative harmony. But after multiple rounds of chemotherapy, this microbial balance unraveled. Diversity plummeted, and communities became dominated by a few hardy survivors, mostly Streptococcus species, while beneficial anaerobes such as Prevotella and Veillonella sharply declined. These shifts suggest that chemotherapy not only reduces bacterial richness but also dismantles functional cooperation within the microbial network.
This loss of microbial diversity may have profound effects on mucosal health. When protective commensals disappear, the mouth’s natural barrier weakens, allowing opportunistic or inflammatory microbes to take over. Reduced production of microbial metabolites and enzymes that normally support tissue repair can leave the mucosa more vulnerable to ulceration and pain. The study found that children who developed more severe mucositis also showed deeper microbial disruption, suggesting a direct link between microbial imbalance and tissue injury severity.
While the research doesn’t yet prescribe a cure, it points toward promising directions—perhaps targeted oral probiotics, microbiome-friendly rinses, or dietary strategies could help restore a healthier microbial balance during treatment. The findings remind us that even in the midst of high-tech cancer care, the smallest inhabitants of our bodies still play a vital role in healing and resilience.
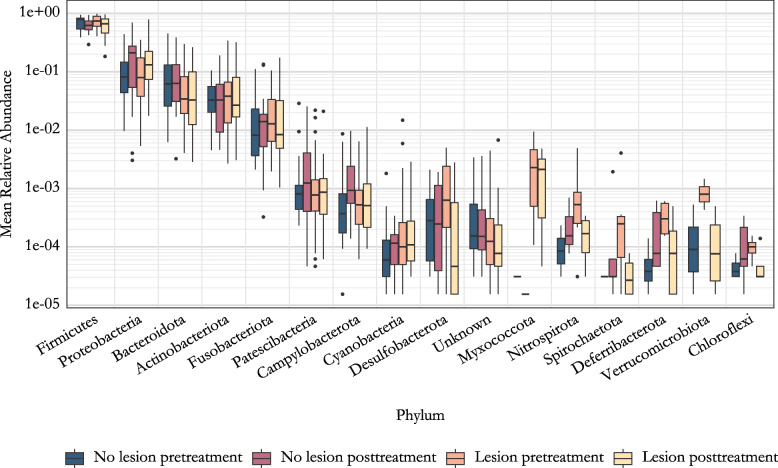
Mean relative abundance of phyla. The data is grouped by the lesion status and the sampling time. Phyla are ordered in decreasing order based on total read count.
Stay tuned to unravel the latest discoveries on dynamic human-microbe interactions!