The Early-Life Microbiome: Impact of Vaginal and C-Section Births
Introduction
At birth, the infant gut is initially colonized by microbes, which gradually evolve into a complex ecosystem known as the gut microbiome. This initial community plays a vital role in shaping their immunity, metabolism and even long term health.
Did you know that the way a baby is born—whether through vaginal delivery or caesarean section—can significantly influence which microbes colonize the gut first? It was believed that babies enter the world completely sterile. Recent studies have challenged this idea, detecting traces of bacterial DNA in the placenta, amniotic fluid, and even in the meconium passed just hours after birth. Babies delivered vaginally are exposed to their mother’s vaginal and gut microbiome, which helps with initial seeding of diverse microbes. On the other hand, babies delivered by C-section are not exposed to vaginal microbes and are often picked from the hospital environment, leading to differences in their early gut composition. These differences may lessen over time, however, early microbial exposures are linked with important outcomes such as immune development, allergy risk, and metabolism.
Vaginal Births
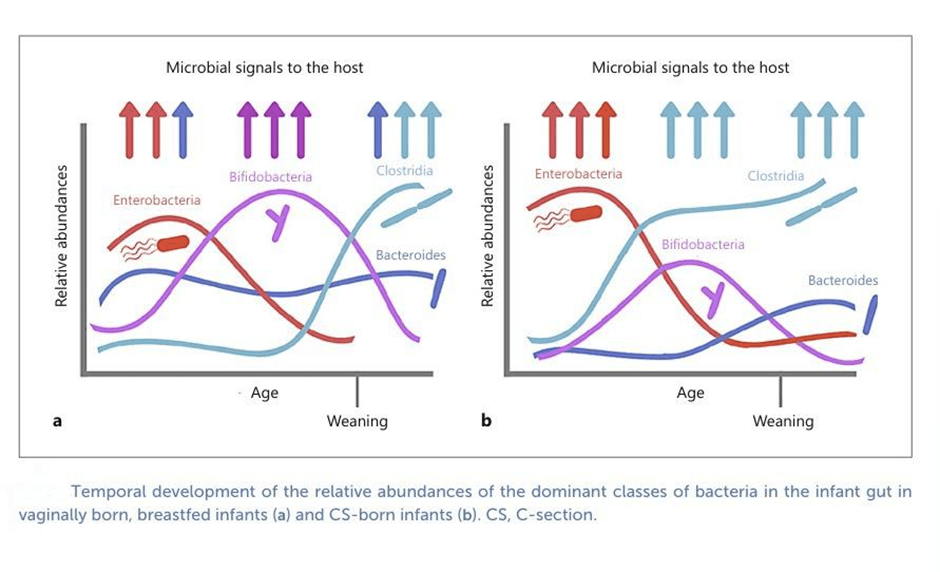
During vaginal births, babies are exposed to their mother’s gut and vaginal microbes during delivery. These include Bifidobacteria and Bacteroides, which are the early colonizers of the infant gut. Bifidobacteria species like B. longum subsp. infantis, B. breve, andB. bifidum are highly specialized in digesting human milk oligosaccharides (HMOs). Meanwhile, Bacteroides species such as B. fragilis, B. vulgatus, and B. thetaiotaomicron act as versatile generalists, capable of breaking down breast milk sugars, host mucus, and later in life, dietary plant polysaccharides, which ensures their long-term stability in the gut. In the earliest days, aerotolerant bacteria like Streptococcus, Staphylococcus, and Enterococcus often appear in meconium (the baby’s first poop) and are soon replaced by Bifidobacteria and Bacteroides -the dominant colonizers of the infant’s gut.
Caesarean Births
C-section babies skip the natural exposure to maternal gut and vaginal microbes. In contrast, their first microbes are often acquired from the hospital environment, and maternal skin. C-section babies typically show lower levels of Bifidobacterium , leading to fewer microbes capable of digesting HMOs and supporting gut health. Notably, Bacteroides species are almost absent, despite their essential role in metabolism of essential nutrients . C-section infants are commonly colonized by hospital-acquired bacteria such as Enterococcus, Enterobacter, and Klebsiella, which are not dominant in the healthy infant gut and may increase susceptibility to pathogens. This altered seeding results in delayed development of the microbial community during the first months of life compared to vaginally delivered infants. Metagenomic analyses reveal that their microbiomes are depleted in pathways linked to carbohydrate, amino acid, and nucleotide metabolism, while enriched in genes associated with lipopolysaccharide (LPS) production, which may raise inflammatory risks. This imbalance has been associated with higher risks of obesity, asthma, allergies, and immune-mediated conditions.
Microbial Functions in Early Nutrition
It’s not just about what microbes are present, It’s about their function too! Vaginally delivered infants, enriched with Bacteroides and Bifidobacteria, show a greater capacity to produce essential nutrients such as B-vitamins (B1, B5, B6, B9, and K2), amino acids, and compounds that strengthen the gut barrier. Likewise, certain Bifidobacteria species, like B. longum subsp. infantis, B. breve, and B. bifidum, are good at breaking down the unique sugars in breast milk, known as human milk oligosaccharides (HMOs). These functions support both nutrient absorption and immune system development during the early life. Research also shows that geography and culture also plays a role on the gut microbiome of the infant. For example, African infants tend to show more Bacilli, while North American infants show more Bacteroidetes.
Supporting Gut Health After Birth
As specified above, there are clear differences in microbiomes between vaginally delivered and caesarean delivered babies, there are few ways to help close the gap :
Breastfeeding: Breast milk is not just food, it is packed with prebiotics and probiotics that help seed the infant gut with Bifidobacteria and other protective microbes. Exclusive breastfeeding for the first six months has been shown to partially restore microbial balance in C-section infants.
Probiotics and Prebiotics: Specifically tailored probiotics (e.g., Bifidobacterium infantis , Lactobacillus rhamnosus strains) may assist in repopulating C-section infants, missing beneficial bacteria. Prebiotics like HMOs, galacto-oligosaccharides, fructo-oligosaccharides in fortified formulas can fuel the beneficial microbes to make them multiply.
Vaginal seeding: This method is still in its experimental stages. Vaginal seeding involves swabbing a C-section baby with the mother’s vaginal fluids to transfer microbes that would normally be passed on during birth. While early studies suggest partial restoration of beneficial microbes, there remain concerns about the transfer of pathogens.
The role of diet and environment: The gut microbial community keeps evolving as the age progresses. Exposure to pets, siblings, and the outdoors also promote a more diverse microbiome. Over the long term, lifestyle like diet, exercise, and the reduced overuse of antibiotics are few important factors in determining a robust gut ecosystem.
Fecal Microbiota Transplantation: In a pilot study conducted, fecal microbiota transplantation from mother to infant shows promising results in restoring the gut microbiota of infants delivered through C-section . In this study a small, screened dose of fecal maternal microbes delivered through breast milk helped reintroduce key bacteria such as Bacteroides and Bifidobacterium. This also reduced colonization by hospital-associated microbes like Enterococcus and Klebsiella. While effective and safe in this study, FMT requires medical supervision due to potential pathogen risks.
- Shriya Chandana S
References
Korpela, K. (2021). Impact of delivery mode on infant gut microbiota. Annals of Nutrition and Metabolism, 77(Suppl. 3), 11-19.
Kim, G., Bae, J., Kim, M. J., Kwon, H., Park, G., Kim, S. J., ... & Kang, B. (2020). Delayed establishment of gut microbiota in infants delivered by cesarean section. Frontiers in microbiology, 11, 2099.
Shi, Y. C., Guo, H., Chen, J., Sun, G., Ren, R. R., Guo, M. Z., ... & Yang, Y. S. (2018). Initial meconium microbiome in Chinese neonates delivered naturally or by cesarean section. Scientific reports, 8(1), 3255.
Wilson, B. C., Butler, É. M., Grigg, C. P., Derraik, J. G., Chiavaroli, V., Walker, N., ... & Cutfield, W. S. (2021). Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: A pilot randomised placebo-controlled trial. EBioMedicine, 69.
Hoang, D. M., Levy, E. I., & Vandenplas, Y. (2021). The impact of Caesarean section on the infant gut microbiome. Acta Paediatrica, 110(1), 60-67.
Pivrncova, E., Kotaskova, I., & Thon, V. (2022). Neonatal diet and gut microbiome development after C-section during the first three months after birth: A systematic review. Frontiers in Nutrition, 9, 941549.
Korpela, K., Helve, O., Kolho, K. L., Saisto, T., Skogberg, K., Dikareva, E., ... & de Vos, W. M. (2020). Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell, 183(2), 324-334.