Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Can We Rebuild the Gut Microbiome After a C-Section? New Research Says, Yes!
Babies born by cesarean section often start life with a very different microbial world in their gut compared to those born vaginally. This difference can have lasting effects on immune development and allergy risk. The new study explored whether it’s possible to “reset” this early imbalance by gently guiding the gut microbiome back toward a healthier trajectory. When cesarean-born infants were given a specific microbiome-supporting program, their gut communities began to resemble those of vaginally delivered babies—richer in beneficial Bifidobacterium species and equipped with genes for digesting human milk oligosaccharides, such as those encoding α-L-fucosidase. These molecular shifts signaled a maturing gut ecosystem that could more effectively process breast milk and support immune training.
Remarkably, the intervention reduced what researchers called the “C-section index”—a microbiome signature that distinguishes cesarean from vaginally born infants—to near-normal levels. This meant the microbial composition and metabolic potential had largely realigned with that of typical early-life colonization patterns. Even more compelling, infants whose gut microbiomes shifted in this way had a lower risk of developing atopic dermatitis, suggesting that microbial restoration may ripple into visible immune benefits.
The findings reinforce how the gut microbiome serves as a biological bridge between birth mode, nutrition, and immune health. By reintroducing key microbial players like Bifidobacterium and restoring milk-metabolizing functions, the study shows that early microbial environments can be reshaped in meaningful ways. It paints a hopeful picture—that targeted microbiome care in the first months of life might one day become a powerful tool to foster healthier immune development in newborns.
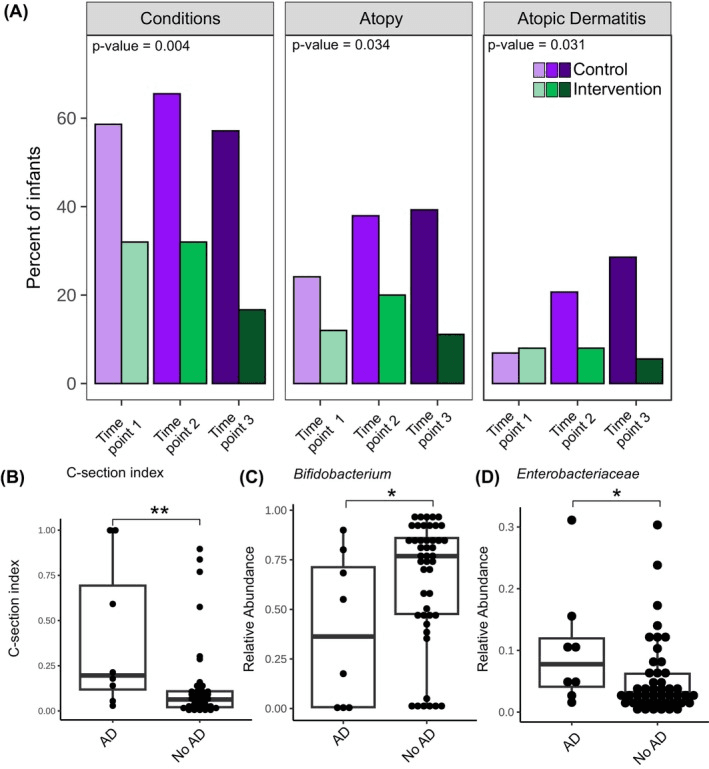
Infants with atopic dermatitis have a higher C‐section index and altered gut microbiome composition. (A) Percentage of infants with different health conditions across time points. The left panel shows the percentage of infants with any condition, and the central panel shows the percentage of infants with atopic dermatitis (AD) and/or any food reaction. (B–D) Association between AD status at time point 2 and (B) C‐section index, relative abundance of (C) Bifidobacterium and (D) Enterobacteriaceae. The Mann–Whitney U test was used to assess statistical significance between the AD and non‐AD groups. p values were adjusted for multiple comparisons using the BH method (q values, 3 tests). *q < .1, **q < .05.
Can Gut Bacteria Help Lift Depression? New Study Offers Clues
The study enrolled adults diagnosed with major depressive disorder (MDD) and gave one group a multi-strain probiotic supplement while another received placebo, with the aim of tracking how the intervention affected gut microbial composition, markers of inflammation, and emotion recognition. What stood out was that the probiotic group showed modest but statistically meaningful shifts in their gut microbiota: increases in beneficial genera such as Bifidobacterium (long associated with gut health) and some Lactobacillus , alongside decreases in pro-inflammatory taxa.These community changes were paired with rises in short-chain fatty acid (SCFA) pathways and improved gut-barrier integrity biomarkers, hinting that the gut ecosystem’s functional capacity had been nudged.
On the mechanistic front, the authors link these microbial shifts to downstream immune and neurobiological effects: greater SCFA production can strengthen the intestinal epithelial layer, reduce leakage of lipopolysaccharide (LPS) into the bloodstream, thereby dampening systemic inflammation and down-regulating the hypothalamic-pituitary-adrenal (HPA) axis hyperactivity often found in depression. The paper reports reduced levels of key pro-inflammatory cytokines in the probiotic arm, suggesting the microbial-immune link was indeed engaged. Interestingly they also assessed emotion-recognition performance: participants receiving probiotics performed better at tasks involving recognising emotional faces, providing an intriguing hint that gut-microbiome shifts might tie into brain-behaviour circuits via immune or metabolic signalling.
What this research underscores is that in MDD, the gut microbiome is not a passive bystander but an active stakeholder: by altering microbial community structure and function (e.g., boosting beneficial Bifidobacterium/ Lactobacillus, enhancing SCFA output, restricting pro-inflammatory taxa), the intervention appears to influence systemic biology and even behavioural outcomes. It’s still early days — sample sizes are modest and the directionality and long-term durability remain to be shown — but this work gives a compelling microbiome-centred glimpse into how gut microbes might play a supporting role in mental-health interventions.
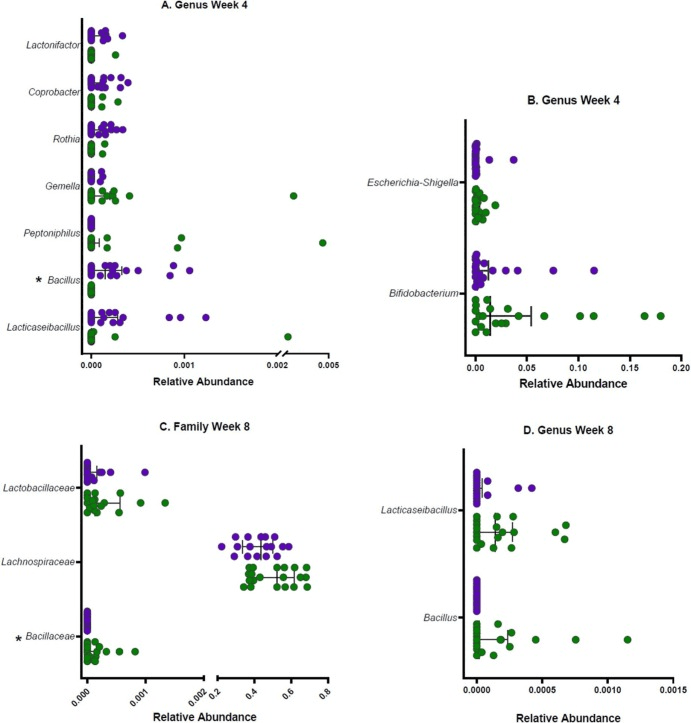
Relative abundance of family and genus-level taxa showing differentiation between the probiotic (green) and placebo (purple) group at A & B. Week 4 (n = 21 probiotic, n = 21 placebo), and C & D. Week 8 (n = 19 probiotic, n = 17 placebo). Taxa that remained significant after FDR correction are annotated with *. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Healing the Gut from Within: How Probiotics and Vitamin D Strengthen the Intestinal Barrier
In a thoughtful exploration of the gut ecosystem, the study titled “Multistrain Probiotics Plus Vitamin D Improve Gut Barrier” dives into how targeted interventions shape microbial allies and reinforce the body’s inner defenses. The trial, conducted in individuals with irritable bowel syndrome without constipation, paired a multi-strain probiotic blend with vitamin D supplementation over twelve weeks. What stands out from a microbiome lens is how this regimen nudged the gut community and its functional output in meaningful ways.
The microbial shifts were modest but telling: beneficial bacteria such as Bifidobacterium and Lactobacillus were enriched, while taxa associated with dysbiosis showed relative declines. Alongside changes in community composition, metabolic markers showed a boost in short-chain fatty acid production—particularly acetate—and fecal lactate rose, suggesting that the resident microbes’ fermentative activity was enhanced. This uptick in microbial metabolism matters because it ties directly into how the gut lining communicates with its microbial residents and the broader immune system.
Perhaps the most compelling layer of the study is how microbial and metabolic shifts aligned with improvements in gut barrier integrity. Biomarkers of permeability—such as zonulin—declined in the intervention group, hinting that a better-equipped microbiome may reinforce the tight-junctions of the intestinal wall, reducing the leakage of pro-inflammatory molecules into circulation. In turn, participants reported improvements in stool form and frequency, suggesting symptomatic benefit. The proposed mechanism unfolds as: nurturing beneficial microbes → elevated metabolite production (SCFAs, lactate) → strengthened gut barrier → less immune activation → reduced gut-related distress.
While the study is exploratory and cannot entirely separate the probiotic from the vitamin D effect, it underscores a broader truth: the gut microbiome is not just a passenger in digestive health—it is an active, modifiable system. By shifting microbial composition and metabolic functions, we may help the gut ecosystem evolve toward a more resilient, balanced state, with ripple-effects on barrier function and symptom relief.
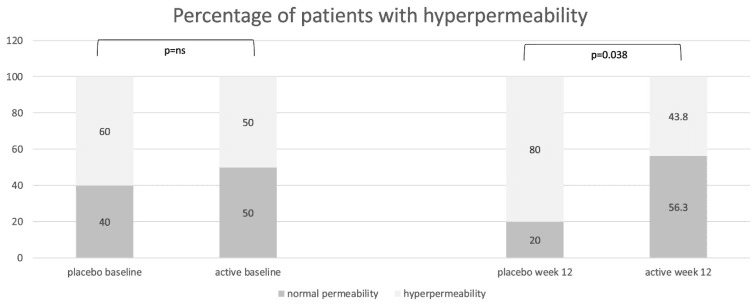
Distribution of patients with normal intestinal permeability (lactulose/mannitol ratio < 0.03) and with increased permeability (lactulose/mannitol ratio ≥ 0.03) in the placebo group and in the active group at baseline and at the end of treatment (week 12), expressed as percentages.
Micronutrients Meet Microbes: How Daily Supplements Subtly Shift the Gut Ecosystem
In a short-term crossover trial among healthy adults, researchers asked whether a standard multivitamin/multimineral (MVMM) supplement could ripple through the gut ecosystem and influence the resident microbial community and its metabolic output. What makes this intriguing from a microbiome perspective is that the supplement doses were close to recommended daily levels (not mega-doses), yet measurable changes emerged.
When participants took the MVMM supplement, the gut microbiome showed modest but specific taxonomic shifts: notably, the abundance of the genus Lachnoclostridium and a group labelled “UCG_005” declined; reductions were also seen in some Actinobacteriota and Bifidobacteriaceae. At the same time, metabolic signals from the microbiome shifted: levels of short-chain fatty acids (SCFAs) such as propionate and butyrate rose, and total faecal sulphide increased — suggesting that even if the bacteria didn’t wildly rearrange, their functional output changed.
What’s compelling is how the study links these microbial shifts to background diet: participants consuming more saturated fat or fewer total carbohydrates exhibited different microbial responses to the MVMM supplement, particularly with regard to sulphate-reducing bacteria like Desulfovibrionaceae. From the mechanistic viewpoint, a plausible chain of events is emerging: micronutrient supplementation tweaks the gut environment (perhaps by altering mineral availability or vitamin-driven cofactor status), this nudges certain microbial taxa or metabolic pathways (e.g., SCFA production, sulphide metabolism), and that in turn may influence host health via gut barrier, immune or metabolic signalling — though the study stops short of connecting to clinical outcomes.
For the science-curious, this study underscores that micronutrients — often thought of as purely human nutritional inputs — also engage our microbial partners. It invites a view of the gut microbiome as a responsive ecosystem that can shift its composition and metabolism in response to subtle nutritional changes. While the trial is short and focused on healthy adults, it opens the door to asking how micronutrient–microbe interactions might matter in longer-term health, disease prevention or personalised nutrition.
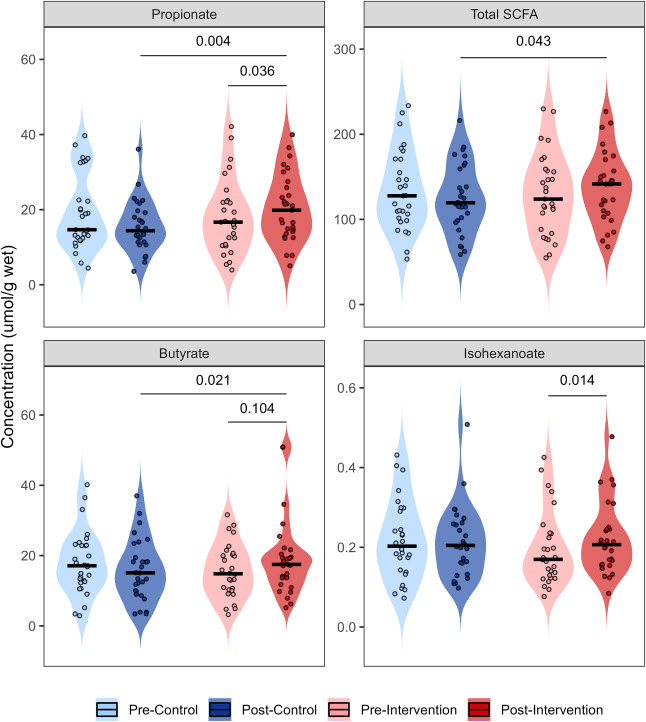
Faecal short chain fatty acid concentration (μmol/g wet) measured via GC-FID before and after 10-days of multivitamin/multimineral supplementation (intervention) and a control period in a cross-over paired design (n = 28). Interaction effects between treatment arm and timepoint were tested using a linear mixed model with box cox natural logarithm transformation. Post hoc analysis was performed using Fisher's LSD test (p < 0.05).
Not Just Fresh Breath: How Toothpaste Shapes the Microbes Beneath Your Gums
When we think of the microbiome we often imagine the gut, but the surfaces around our teeth host their own rich microbial communities—and this study looked closely at the subgingival zone (the region under the gum line) to see how a toothpaste containing the antimicrobials cetylpyridinium chloride (CPC) and cymenol might shift that ecosystem. Over six weeks of routine use in people with gingival inflammation, the researchers found that the overall diversity and broad structure of the subgingival microbiome remained stable—meaning the microbial ecosystem did not collapse or swing wildly.
Peering deeper into specific taxa, they saw that in the CPC-cymenol group the relative abundance of Fusobacterium nucleatum (a species often associated with periodontal dysbiosis) declined significantly, and Aggregatibacter decreased more in this group than in the fluoride toothpaste group. Meanwhile, organisms classically implicated in more advanced periodontal disease—such as Porphyromonas gingivalis, Prevotella intermedia and Tannerella forsythia—were unaffected in either group.
From a microbiome mechanisms view this suggests that the toothpaste regimen, while not radically altering the subgingival community, nudged it in a slightly ‘healthier’ direction by suppressing some of the key “bad actor” taxa without disturbing overall microbial richness or evenness. For the science-curious, it reinforces a valuable insight: the microbial ecosystems in and around our bodies are resilient, and subtle interventions—like toothbrushing with a specific formula—can produce measurable taxonomic shifts without wholesale disruption. While the site here is the mouth and not the gut, the principle holds: microbial balance doesn’t require massive upheaval; sometimes easing back the abundance of the more problematic species is enough to swing things toward less inflammation-prone states. The study is pilot in nature and limited to six weeks, but it adds to our growing appreciation of how daily hygiene products influence our intimate microbial partners.
Comparison of the subgingival communities between toothpastes. The analysis did not show any difference in α-diversity metrics at the phylotype level. Richness (observed amplicon sequence variants, ASVs) (A) and evenness (Pielou’s Evenness index) (B). Treatment groups: CPC/cym, toothpaste with cetylpyridinium chloride (CPC) and cymenol (cym), as main active ingredients; MFP, toothpaste with sodium monofluorophosphate (MFP)
