Once upon a time, being chubby and obese was a mark of affluence. As in earlier times, food was often insufficient, and URL: https://www.bugspeaks.com/blog/being-chubby---mark-of-affluence-or-aberration Once upon a time, being chubby and obese was a mark of affluence. As in earlier times, food was often insufficient, and famines were common obesity meant prosperity. But the world changed, and food became abundant in many regions of the world. So, what was once a mark of prosperity, has now become a sign of an unhealthy lifestyle. Obesity involves the accumulation of a considerable amount of body fat, which intensifies the risk of additional health hazards—for example, diabetes, heart disorders, liver diseases, high blood pressure, and certain cancers. Yet, over the past few decades, obesity and related co-morbidities have reached epidemic magnitudes. People continue to eat more. The modern industrial world considers it a [crisis of communal](https://www.nejm.org/doi/full/10.1056/NEJMoa1614362) wellbeing. Generally, obesity is a result of an amalgamation of a few factors like heredity, lifestyle, and environment. Among the environmental elements, the gut microbiome flourishing in our bodies seems to play a vital role.  **Our gut microbiome** As our gut plays home to a vast and diverse array of microorganisms, they, in return, play an essential role in several vital processes, like, digestion, metabolism, and energy production. Five bacteria, Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobia, are predominant in the gut flora of an average healthy individual, with Bacteroidetes and Firmicutes, making around [90%](https://www.ncbi.nlm.nih.gov/pubmed/15505215) of the total. Our gut flora, while regulating numerous bodily processes, also influences the production, metabolism, storage, and [breakdown of lipids](https://www.ncbi.nlm.nih.gov/pubmed/31391921). Lipids are the small molecules that also makeup fat. **How microscopic gut microbes influence macroscopic bodyweight?** Like in other metabolic diseases, the gut microbiota in over-weight people shows misbalance as compared to normal [healthy individuals](https://www.ncbi.nlm.nih.gov/pubmed/15505215). Dysbiosis, the imbalance in the proportion of good and bad gut microbes, causes varied digestion of otherwise indigestible complex carbohydrates. Such digestion of carbohydrates contributes to the risk of developing obesity. Digestive juice of the upper GI tract cannot digest complex dietary carbohydrates, including polysaccharides, oligosaccharides, and starches of plant origin. Only the microbiota harbouring our gut can ferment them and produce Short Chain Fatty Acids (SCFAs). [SFCAs](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5867888/) act as the primary energy source to support both the microbial growth and the gut cells. It also regulates optimal lipid and cholesterol metabolism besides colonic immunity. But, when you continuously eat food low in complex carbohydrates, it alters gut pH and modifies the structure and metabolism of gut microbiota—reduced dietary carbohydrate results in the reduction of total SCFA. Also, an alteration in gut microbiome profile characterized by decreased Roseburia, the Eubacterium, and Bifidobacteria occurs. Additionally, a protein-rich diet with a low proportion of carbohydrates also alters gut microbiome [composition](https://www.ncbi.nlm.nih.gov/pubmed/24999296). The ratio of the abundance of Gram+ and Gram− bacteria gets ultimately reverses in the gut of both the obese and [anorexic people](https://www.ncbi.nlm.nih.gov/pubmed/28636668). **Gut crisis when good microbes crash** Health remains the most valuable currency. If not realized on time, it can cost you a lot of your actual money. So, how the good microbes go up and down in an obese gut? Gut microbiome composition is extremely diverse between healthy individuals, lean, and overweight ones, characterized by the low [bacterial richness](https://www.nature.com/articles/nature12506) in obese subjects. High numbers of Firmicutes and low numbers of [Bacteroidetes](https://www.ncbi.nlm.nih.gov/pubmed/23719380) occur in obese people. The proportion of Bacteroidetes increases with low-calorie diet-based [weight loss](https://www.ncbi.nlm.nih.gov/pubmed/17183309), thus, suggesting a tight association between the gut microbiota and body [weight regulation](https://www.ncbi.nlm.nih.gov/pubmed/6834773). Less diversified gut flora during obesity becomes inefficient in consuming energy produced from [metabolic processes](https://www.ncbi.nlm.nih.gov/pubmed/23478685). Altered gut flora in overweight people thus causes amplified production of energy from food. It also promotes the storage of fats by encouraging the [formation of biological](https://www.ncbi.nlm.nih.gov/pubmed/27703805) products required for the same. Obesity-induced transformed intestinal flora also produces bacterial endotoxins, which pass into our circulation and cause inflammation and insulin resistance. Finally, leading to enhanced fat deposition and related [complications](https://www.ncbi.nlm.nih.gov/pubmed/27616451). Alterations of the gut microbiome in initial life also boost the onset of obesity due to altered mechanisms regulating [satiety/appetite](https://www.ncbi.nlm.nih.gov/pubmed/25126780). Gram-negative bacteria, abundant in the gut of obese people, damage the gut epithelium and enhance the permeability of the intestinal wall. As a consequence, loads of bacterial endotoxins enter the blood circulation stimulating the production of molecules associated with [obesity](https://www.ncbi.nlm.nih.gov/pubmed/20508158). Moreover, excessive gut barrier disruption can also result due to the activation of metabolic pathways that affect adipose tissue. This also aggravates the initial disorders and leads to a vicious cycle of [detrimental processes](https://www.ncbi.nlm.nih.gov/pubmed/20664638). **Giving the microbes healthy nutrition** One can correct dysbiosis induced obesity by tracing the path back to healthy gut microbiota. Just correcting the proportions of healthy microbes can help you become lean. One can reform gut dysbiosis by several months of the regular low-energy diet, including intake of some micronutrients, prebiotics, and probiotics. Altered diet impacts the regulation of gene expression of the liver, muscle, and adipose tissue along with the alteration of [gut microbiome composition](https://www.ncbi.nlm.nih.gov/pubmed/25361999). Regular intake of probiotics improves gut health by restoring the gut epithelium, healthy bloodstream, and controlling [local inflammation](https://www.ncbi.nlm.nih.gov/pubmed/28792488)19. Antioxidants present in tea, like oolong tea polyphenols (OTP), mainly increase gut bacterial biodiversity with a higher number of Bacteroidetes and a low number of Firmicutes. They are thereby helping your gut experience the healthy flow of SCFAs once again. Intake of another prebiotic, [galacto-oligosaccharide (GOS)](https://www.ncbi.nlm.nih.gov/pubmed/29465126), encourages the abundance of Bacteroides, Ruminococcaceae, and Oscillibacter. It not only reduces the production of harmful bacterial metabolites but also brings down blood triglyceride levels. Thus, probiotics and prebiotics like, GOS and OTP can be used to prevent obesity-related metabolic disorders through modification of the [intestinal microbiota](https://www.ncbi.nlm.nih.gov/pubmed/29355278). Additionally, regular physical exercise also helps to lose weight. It also restores gut health by encouraging beneficial gut flora, which synthesizes SCFAs and controls the [local inflammation](https://www.ncbi.nlm.nih.gov/pubmed/31380886). Another therapeutic option to treat obesity is through various surgical techniques, known as Bariatric surgery, which find a relation with alteration of gut flora and weight reduction and other [health benefits](https://www.ncbi.nlm.nih.gov/pubmed/28359101). **Gut microbiome test: a helpful tool** The fact is that even modest weight loss can improve or prevent obesity-associated complications. For that, it is a prerequisite to have a ‘Gut microbiome test.’ It identifies your unique microbiome. State-of-the-art, ‘DNA sequencing technology,’ and algorithm-based data acquisition are used in this process. A prescription diet is formulated comprising of probiotics, and prebiotics based on the test results. Dietary changes increased physical activity, and lifestyle is the key to lose weight. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Gut Microbiome in Atherosclerosis: Unclogging the Bloody Pipes Author: BugSpeaks Published: 2020-07-10 Category: Microbiome and Disease Meta Title: Gut Microbiome in Atherosclerosis: Unclogging the Bloody Pipes Meta Description:
All the blood in the human body flows through a network of arteries, just like the drainage system in house. When not cl URL: https://www.bugspeaks.com/blog/gut-microbiome-in-atherosclerosis:-unclogging-the-bloody-pipes All the blood in the human body flows through a network of arteries, just like the drainage system in house. When not cleaned regularly, sometimes the pipes in the system get blocked as the dirt and filth collect. Similarly, your arteries may get blocked if not cared for properly, as the fatty matter deposits in them. The arteries become narrow leading to the grave disease called atherosclerosis. So why the arteries get clogged? The sedentary lifestyles are one to blame for. Moreover, elevated blood cholesterol, high blood pressure, diabetes, obesity, family history, an unhealthy lifestyle, and abnormal gut microbes are blamed for the [disease](https://www.ncbi.nlm.nih.gov/pubmed/23618829). Yes, you read it right. The microbes residing in your gut, which make up the gut microbiome can also place you at atherosclerosis risk. Your gut microbiome plays a vital role in controlling the metabolism and health of organs. Hence, it is quite relevant to explore the purpose of this diverse ecosystem of microorganisms in causing atherosclerosis.  **Gut microbiome dysbiosis: Cause of blockage** Atherosclerosis is a classical chronic inflammatory disease. The healthy gut flora supports lipid (small molecules which makeup fat) metabolism and prevents the development of lesions on [arteries](https://www.nature.com/articles/s41467-017-00900-1). But [gut dysbiosis](https://www.ncbi.nlm.nih.gov/pubmed/23212374), characterized by maladaptation of gut flora, proves to be a significant player in the event of atherosclerosis. Under this condition, a relatively lower abundance of beneficial microbes such as Roseburia and Eubacterium, and overpopulation of harmful ones such as Collinsella occurs. Not only this, but dysbiosis also damages the [gut wall](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4590619/) and impairs its integrity. It gives the microbial components like lipopolysaccharide (LPS) and peptidoglycan a chance to escape into the blood circulation and reach arteries. Upon entering the arteries, these components cause inflammation resulting in the development of [atherosclerosis](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4456003/)9. **What all microbial materials block your arteries?** It is not only the components of gut microbes wreaking havoc in arteries. Specific metabolites produced by gut microbes also control inflammation and atherosclerosis. These metabolites include short-chain fatty acids (SCFAs), methylamines, polyamines, trimethylamine N-oxide (TMAO), and secondary bile acids (BAs). All of them play an essential role in [fat metabolism](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579652/#!po=34.0000). SCFAs arise from microbial fermentation of starches and complex dietary fibres in the intestine. Taking part in further metabolic events in the body, the SCFAs inhibit the accumulation of lipid and inflammation in the arterial wall. Also, [SFCAs](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579652/#!po=34.0000) induce secretion of gut hormones, which reduce food intake. But in a dysbiotic gut, [butyrate](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579652/#!po=34.0000), a critical SCFA occurs in low amounts, as butyrate-producing bacteria such as Roseburia, occur in small numbers. Likewise, the inflammatory insults on the arterial walls increases. TMAO comes from the oxidation of trimethylamine (TMA), a product of gut microbe activity in the liver. Varying compositions of bacteria pose differential abilities to generate TMAO. Thus, a dysbiotic gut containing higher numbers of TMA-producing bacteria can produce higher levels of [TMAO](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579652/#B58-nutrients-09-00859). A higher level of TMAO causes atherosclerosis and larger [plaque area](https://www.nature.com/articles/nature09922). Even low levels of circulating TMAO attenuates atherosclerosis. Studies have found a correlation between TMAO levels and certain human gut microbes’ types. For example, [Prevotella](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5579652/#B59-nutrients-09-00859) finds a relation with high TMAO levels while Bacteroides produce lower levels. Bile acids (BA) form another group of metabolites produced from cholesterol. BA helps absorb dietary lipids and fat-soluble vitamins. Gut microbiota-derived enzymes [convert](https://www.ncbi.nlm.nih.gov/pubmed/26939849) primary BAs into secondary BAs. Secondary BA influences cholesterol metabolism in host and energy expenditure. But when in a dysbiotic gut, the enzyme activity reduces the gut does not reabsorb secondary BAs that occur in large numbers. So, these secondary BAs enter the blood circulation, impair elimination of cholesterol, and signal deposition of fats, leading to the development of atherosclerosis. [Methanobrevibacter smithii](https://www.ncbi.nlm.nih.gov/pubmed/22972297), Clostridium, and Enterococcus find an association with reduced enzyme activity.14 Additionally, the [BA receptors](https://www.ncbi.nlm.nih.gov/pubmed/27045028) in inactivated form inhibit atherosclerosis formation by reducing macrophage inflammation and lipid loading15. **How to manage and correct the blockage??** To correct the blockage, we just need to treat the source, meaning the gut microbiome. If one can restore the balance in the gut, the balance in arteries may also be restored. Therefore, the intestinal microbiome offers a new potential target for the treatment of atherosclerosis. **Probiotics** Just like pouring a cleaning solution down the drainage pipe helps remove collected material, similarly, pouring probiotics in your body can help. [Probiotics](https://www.ncbi.nlm.nih.gov/pubmed/25529048) contain beneficial live microorganisms. Ingestion of viable microorganisms, like, Lactobacillus rhamnosus GR-1, helps hydrolyse bile salts, lower blood cholesterol, and reduce the risk of developing atherosclerosis. In essence, the beneficial microbes can help restore healthy metabolism and removal of plaques in arteries. **Healthy diet** Increased consumption of [whole-plant](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4315380/) foods, including fruits, vegetables, and wholegrain [cereals](https://www.ncbi.nlm.nih.gov/pubmed/22360862), helps modulate the gut microbiota. These foods share an inverse correlation with the risk of atherosclerosis development. Antioxidant abundant plant polyphenols prevent the oxidation of low-density lipoprotein. [Cranberry extract](https://www.ncbi.nlm.nih.gov/pubmed/25080446) (CE), a rich source of polyphenols, reduces fatty deposits on arterial walls. Additionally, the intake of CE increases the number of beneficial bacterium Akkermansia. It helps reduce fat storage and the size of plaques. Intake of fibre-rich wholegrain foods, on the other hand, associates with lower blood glucose and total lipid. Dietary fibres also aid in the proliferation of [gut-friendly flora](https://openheart.bmj.com/content/6/1/e000993#ref-48): Bifidobacterium, Lactobacillus, and Enterococcus. Thus, a diet based on healthy foods promotes a happy and healthy microbiome. Strategies that modulate the gut microbiota and their metabolic activities by whole-plant foods, probiotics, and prebiotics may be at the base of healthy eating pyramids. They can decrease the risk of atherosclerosis and related co-morbidities21. The [modern-day diet](https://www.ncbi.nlm.nih.gov/pubmed/22468338) remains rich in high calorie processed food with high sugar and fat content. While raw and whole food and dietary fibre and fermentable substrates are relatively low, this type of urban diet with high saturated and trans fatty acids relates to the depletion of good metabolic fuels. All this negatively alters the gut microbial profile contributing to increased cholesterol levels[.](https://openheart.bmj.com/content/6/1/e000993#ref-53) A shift towards a plant-based diet may confer protective effects against atherosclerosis by promoting protective factors and reducing harmful ones. **Cow Milk** Drinking' [bovine colostrum'](https://www.ncbi.nlm.nih.gov/pubmed/22236001), the milk produced by cow up to a few days after the birth of calf remains beneficial. The colostrum contains anti-LPS compounds, which alleviate hyperlipidaemia and atherosclerosis. These compounds also help in healing 'leaky' gut mucosal membranes by removing harmful microbial toxins circulating in the blood. Thus, these compounds reduce local inflammation and prevent disease progression. **Surgery & Exercise** [Bariatric procedures](https://pubs.acs.org/doi/abs/10.1021/pr400748f), mainly used to lose weight, also help improve obesity-associated metabolic disorders such as atherosclerosis. It also helps in the alteration of the gut microbiota as the body experiences a significant shift in metabolic requirements. Regular physical exercise also alters gut microbial composition. [Exercise](https://www.ncbi.nlm.nih.gov/pubmed/28862530) increases the number of SCFA producing bacteria, thus helping in preventing atherosclerosis and related complication[25](https://openheart.bmj.com/content/6/1/e000993#ref-64). **Summing Up** Atherosclerosis is a prevalent disease, and surely, you will accept that the health of your gut microbiome and your lipid metabolism are entangled. So, to cure it, you need a 'Gut Microbiome Test.' It uses sophisticated 'DNA sequencing technology' in stool samples to give a snapshot of the exclusive assortment of microorganisms present in your gut. Then this data will then be used to prepare your individualized diet plan, comprising probiotics, prebiotics, and regular physical exercise. In this way, you can tackle your atherosclerosis. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Urinary Metabolites & Microbiome Author: BugSpeaks Published: 2020-06-23 Category: Microbiome and Disease Meta Title: Urinary Metabolites & Microbiome Meta Description:
"I'm not just your PEE but your METABOLIC INDEX"
From the t URL: https://www.bugspeaks.com/blog/urinary-metabolites-&-microbiome "I'm not just your PEE but your METABOLIC INDEX" From the time immemorial 'PEE or Urine' has been regarded as a vital biofluid with substantial biological information and details and an indicator to the one’s "Pink of Health"and "Striking vigour". Does it remind you of our Grandma’s Traditional knowledge and Home remedies …?? Of Course!! It is well established fact that the properties of urine have long been regarded as a yardstick for wellness, a fact well substantiated in its mention in scriptures of Shushrutha in Indian Alternative Medicine like Ayurveda and Naturopathy. The use of urine in medical analysis dates to ancient Egypt, Byzantine Era, and the middle ages. Hippocrates largely legitimized the medical practice of Uroscopy, even the use of urine colour wheels for diagnosis of various diseases was much in vogue. Thus, the Colour, Odour and the Frequency of urination can give a hint of what is going on in the body... so let’s face it... Do check it out before you flush it out of sight...  Urine being the favoured biofluid clinically is a storehouse of metabolic by products such as Urea, Uric Acid, Creatinine, Inorganic Ions, several pigments and the remnants of food, drinks, drugs, chemicals, environmental contaminants, and microbial intermediates. The presence of significant levels of proteins and sugars indicate potential health concerns. However, it is sterile, obtained in large volumes and largely free from proteins and lipids. Being chemically diverse, it is always a challenge to characterise chemical composition of the “Water of Life” as it is also referred to, while the newly advent technologies like NMR Spectroscopy, Gas Chromatography Mass Spectroscopy, High Performance Liquid Chromatography, Direct Flow Injection Mass Spectrometry, Inductively coupled plasma mass spectrometry comes in handy yielding a comprehensive, quantitative and metabolome wide elucidation of urine. These multiple metabolic platforms have helped to identify and annotate around 3079 urinary metabolite species in total. Thus, Urine stands foremost as the highly preferred biological sample for metabolomics research. Metabolomics stand as relatively new field in comparison with its sister “omics”” (Genomics and Proteomics) yet highly promising, for it being “the study of Metabolites” which are the downstream products of various genome wide and Proteome wide interactions, is essentially a sensitive measure of an organism’s phenotype. With its far-reaching impacts as Disease Biomarkers and as Indicators of Environment gene Interactions and Drug Toxicity ,metabolomics warrants its potential by taking a step further in predicting the microbial health of an individual. **Microbial Metabolomics:** The Gut Microbiota consists of 10 trillion microbial cells and is a dynamic stage for small molecules and other bioactive compounds that can trigger both host metabolic and immune interplay. The microbial ecosystems in the gut contain about 1000 different bacterial species with a close-knit homeostasis established among them through cell-cell signalling and production of anti-microbial peptides. Along with achieving community dynamics with other neighbouring microbes, the gut microflora also communicates with the human host either in a symbiotic or deleterious fashion which in turn contributes to human diseases. With the advances in metagenomics and Metabolomics ,particularly 16sRNA amplicon sequencing and Shot Gun Sequencing has led to the identification of previously unidentified members of gut microbial community. **Urinary Microbial Metabolites as Disease Markers:** The diagnosis of gastrointestinal (GI) diseases is usually based on techniques such as upper or lower GI endoscopy, while extremely sensitive and specific non-invasive diagnostic or screening tools are usually lacking. Hence, it is imperative for the development of novel technologies for the detection of these disorders at an early stage. In this regard, Urinary Microbial Metabolites as the non-invasive biomarkers of the gastrointestinal disorders is highly convincing. **Microbial Metabolites in IBD:**  Hallmark of IBD is the microbial dysbiosis characterized by the decrease in commensals versus the pathogenic organisms. Commensals are protective in function against the pathogens through the secretion of bioactive substances as SCFAs (Short Chain Fatty Acids) which maintains the epithelial integrity and improves the barrier function are produced from the microbial fermentation of Dietary fibre by anaerobic microbiota in the intestine and colon. SCFAs activate specific G protein coupled receptors specifically GPR 43 and GPR41 which in turn mediate cell activation, proliferation, differentiation, and production of other bioactive molecules. The other mechanisms of SCFA include inhibition of HDAC, through improving the acetylation of histones impacts several genes and proteins. SCFAs also regulate T-cell differentiation and response through mTOR activation. There is a marked depletion in urinary microbial metabolites such as SCFA, Methylamine, Trimethylamine, Fucose, Citrate, Hippurate, Taurine, Succinate, 2-Hydroxy-Isobutyrate levels during the progression of IBD.  **Microbial Metabolites in Neurological Behaviour:** Tryptophan ,the least abundant amino acid in proteins is necessary for protein biosynthesis and synthesis of neurotransmitters like Serotonin and Melatonin. The metabolic activity of Gut microbiota influences the availability of Tryptophan, consequently the serotonergic signalling, which further emphasises the significance of gut microbiota in modulation of human behaviour. Tryptophan is metabolised by microbial enzymes to a range of indole derived intermediates such as Indole 3 propionate and Indole-3-Acetate which are characterized in the biofluids. Tryptophanase in Indole producing bacteria brings about the formation of Indole from Tryptophan, further, Oxygenases in the non-indole producing bacteria catalyse the production of Indole-3-Propionate (IPA). The commensal bacteria primarily Clostridium sporogenes largely demonstrates the synthesis of IPA from Tryptophan. The detection of the novel metabolites like Methyl Indole 3 Acetate and Methyl Indole 3 Propionate in urine warrants the potential role of gut microbiota on serotonin related gut brain axis disorders. **Arabinitol:** Several Candida species like C. albicans, C. tropicalis. C. parapsilosis, C. pseudotropicalis, C. lusitaniae, C. guilliermondii produce D. Arabinitol in cultures. The elevated D-Arabinitol and L arabinitol ratios confers a serious threat to the susceptibility to Invasive candiasis (grave infection caused by the species of Candida) giving early warning signs for a potential candidate to set out on an anti-fungal therapy at the earliest. **Microbial Phenolic metabolites & Inflammation:** Several low molecular weight phenolic acids of microbial origin are produced in the blood of septic patients at high levels. Bifidobacteria, lactobacilli, produced in vitro considerable amounts of phenyllactic and p-hydroxyphenyllactic acids, Clostridia s. produced great quantities of phenylpropionic and p-hydroxyphenylpropionic acids, p-hydroxyphenylacetic acid was produced by Pseudomonas aeruginosa and Acinetobacter baumanii; and benzoic acid, by Serratia marcescens. Influence of Phenolic acids on ROS production in mitochondria and neutrophils has been established. Low-molecular weight phenolic acids of microbial origin participate in the regulation of the ROS production in both the circulation and tissues, thereby affecting the level of oxidative stress in sepsis and inflammatory syndrome... The mechanism of sepsis includes the excessive production of defensive and inflammatory responses like increased generation of ROS,NO and inflammatory cytokines. The level of oxidative stress is crucial in the genesis and outcome of sepsis. Pathogen-associated molecular patterns such as bacterial lipopolysaccharides and lipoteichoic acids, which are recognized by immune cells and initiate an acute response to pathogens; microbial phenolic acids may be assumed to regulate the magnitude of this response. Phenolic acids act on targets that produce ROS in the organism. The mechanism underlying the action of Phenyllactic acid and Para Hydroxy Phenyllactic acid is related to scavenging superoxide anion. On the contrary, cinnamic and benzoic acids do not act as ROS scavengers but exhibit the prooxidant activity, interacting with thiol groups. Presumably, their effect can be explained by the inhibition of NADPH-oxidase. Some phenolic acids, which decrease ROS production in both mitochondria and neutrophils, can play a role of natural antioxidants. By affecting neutrophils, they retard the immune response, whereas while acting on mitochondria, they prevent the development of multiple organ failure or reduce it. Thus, microbial metabolites can either increase or reduce the inflammatory syndrome. The high levels of phenyllactic and p- hydroxyphenyllactate acids correlate with the development of sepsis and the risk of lethal outcome. **References:** 1\. Bouatra S, Aziat F, Mandal R, Guo AC, Wilson MR, Knox C, et al. (2013) The Human Urine Metabolome. PLoS ONE 8(9): e73076. [https://doi.org/10.1371/journal.pone.0073076](https://doi.org/10.1371/journal.pone.0073076) 2\. Martinez, K. B., Leone, V., & Chang, E. B. (2017). Microbial metabolites in health and disease: Navigating the unknown in search of function. Journal of Biological Chemistry, 292(21), 8553–8559. doi:10.1074/jbc.r116.752899 3\. Knights D, Lassen KG, Xavier RJ : Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome Gut 2013;62:1505-1510. 4\. Sarosiek I, Schicho R, Blandon P, Bashashati M. Urinary metabolites as noninvasive biomarkers of gastrointestinal diseases: A clinical review. World J Gastrointest Oncol. 2016;8(5):459-65. 5\. [Tereza Pavlova](https://www.sciencedirect.com/science/article/pii/S0003267017309595?via%3Dihub#!), [Veronika Vidova](https://www.sciencedirect.com/science/article/pii/S0003267017309595?via%3Dihub#!), [Julie Bienertova- Vasku](https://www.sciencedirect.com/science/article/pii/S0003267017309595?via%3Dihub#!), [Petr Janku](https://www.sciencedirect.com/science/article/pii/S0003267017309595?via%3Dihub#!), Martina Almasi, [Jana Klanova](https://www.sciencedirect.com/science/article/pii/S0003267017309595?via%3Dihub#!), [Zdenek Spacil](https://www.sciencedirect.com/science/article/pii/S0003267017309595?via%3Dihub#!), [Analytica Chimica Acta](https://www.sciencedirect.com/science/journal/00032670), [Volume 987](https://www.sciencedirect.com/science/journal/00032670/987/supp/C), 22 September 2017, Pages 72-80 6\. Beloborodova N, Bairamov I, Olenin A, Shubina V, Teplova V, Fedotcheva N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J Biomed Sci. 2012;19(1):89. Published 2012 Oct 12. doi:10.1186/1423-0127-19-89 --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Depressed gut, Depressed mind Author: BugSpeaks Published: 2020-02-27 Category: Microbiome and Lifestyle Meta Title: Depressed gut, Depressed mind Meta Description:
We all might have experienced bouts of depression some time in our life. Depression, a common psychiatric illness, presents a demo URL: https://www.bugspeaks.com/blog/depressed-gut,-depressed-mind We all might have experienced bouts of depression some time in our life. Depression, a common psychiatric illness, presents a demoralized state wherein both the mind frame and body get affected. It affects feelings and judgmental capabilities and compromises the [quality of life](https://www.guilford.com/books/Handbook-of-Depression/Gotlib-Hammen/9781462524167). Usually, depressed people are unable to concentrate and feel low all the time. Consequently, their performance in different areas such as academics or profession drops, causing additional stress. In extreme cases, it may lead to severe hopelessness and self-harm, with the thought of [self-destruction and death](https://www.who.int/topics/mental_health/factsheets/en/). Depression, stress, and anxiety all are interrelated and characterized by alteration in food habits and sleeping patterns. Stress and depression influence the release of cortisol (stress hormone). [Cortisol](https://www.ncbi.nlm.nih.gov/pubmed/22972297) modifies gut immunity, ultimately altering the microbial population in gut. It causes a significant modification in the gut microbial diversity, in terms of intensification or decline of individual species. However, the introduction of different genera or the complete removal of specific bacterial genera is [not observed](https://www.ncbi.nlm.nih.gov/pubmed/27005587).  **Role of Gut Microbiome** The gut flora of clinically depressed individuals differs in terms of the abundance and diversity of the common microbes. These include _Bacteroidetes_, _Firmicutes_, _Proteobacteria,_ and _Actinobacteria_. Further, a reduced abundance of bacteria metabolizing carbohydrates also occurs in individuals with depression as compared to healthy ones. These include [_Bifidobacterium_](https://www.ncbi.nlm.nih.gov/pubmed/24742328), [_Faecalibacterium_](https://www.researchgate.net/publication/320413695_Prebiotic_potential_of_pectin_and_pectic_oligosaccharides_to_promote_anti-inflammatory_commensal_bacteria_in_the_human_colon), _Bacteroides_ _Coprococcus_, _Dialister,_ and [_Ruminococcus_](https://www.ncbi.nlm.nih.gov/pubmed/20581222). While several other bacterial species, playing a pivotal role in protein and amino acid metabolism, remain over-populated in depressed subjects. Such bacteria involve [_Clostridium_](https://www.ncbi.nlm.nih.gov/pubmed/24499426), [_Klebsiella_](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5682003/), [_Parabacteroides_](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4850288/), [_Streptococcus_](https://www.ncbi.nlm.nih.gov/pubmed/24499426), _Oscillibacter,_ and _[Alistipes](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5682003/)._ **Dysbiosis** Depression and gut dysbiosis find a [deep connection](https://www.researchgate.net/publication/322937907_Gut_microbiome_and_depression_What_we_know_and_what_we_need_to_know) as the gut and brain share a two-way communication. The reduced richness and assortment of the vast array of the microbes in our gastrointestinal tract also influence our mind frame and [mood swings](https://www.ncbi.nlm.nih.gov/pubmed/27005587). Such correlation has been indicated by a small number of studies where dysbiosis found a relation with abnormalities in behaviour observed in [neurodevelopmental disorders](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6378305/#B25). **Production of Neurotransmitters** Apart from digesting and fermenting food and producing vitamins, gut flora also acts as a source of [neurotransmitters](https://www.ncbi.nlm.nih.gov/pubmed/22612585), like, serotonin, gamma-aminobutyric acid (GABA), glutamate, dopamine, and noradrenalin. These metabolites play an essential role in maintaining [gut immunity](https://www.ncbi.nlm.nih.gov/pubmed/25505251) and prevent the [colonization of the gut](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4083503/) by pathogens. Reduction in levels of serotonin, noradrenaline, dopamine and malfunction of the [glutamate-GABA system](https://www.ncbi.nlm.nih.gov/pubmed/23064760), find a close connection with depression and changed gut biology. Moreover, GABA producing, _[Bifidobacterium](https://www.ncbi.nlm.nih.gov/pubmed/27512962),_ also occur in a low number in depression. On the other hand, environmental factors, the well-being of gut and relative diversity of gut microbes actively modify microbes which regulate [neurotransmitter](https://www.ncbi.nlm.nih.gov/pubmed/29286051) levels. Another neurotransmitter serotonin and tryptophan metabolites if unused, enter the [circulation](https://www.nature.com/articles/mp201277) and influence [behavioural responses](https://www.ncbi.nlm.nih.gov/pubmed/27794467). The [gut microbiota](https://www.ncbi.nlm.nih.gov/pubmed/28053341) also modifies brain function indirectly through changes in inflammatory states and immune status of the gut. **Carbohydrate metabolism** Edible fibers found in cereals, fruits, vegetables, nuts, lentils and grains are indigestible. Our gut bacteria convert the long edible fibers to short chain fatty acids (SCFAs). These [SCFAs](https://www.ncbi.nlm.nih.gov/pubmed/24997036) play an essential role in gut biology by acting as an energy source for intestinal cells and the microbes in gut. Interestingly the SCFAs inhibit enzymes which function as immunosuppressive, [anti-inflammatory](https://www.ncbi.nlm.nih.gov/pubmed/25170769), and [anti-depressive agents](https://www.ncbi.nlm.nih.gov/pubmed/26488817). Resultantly, [low amounts of SCFA](https://www.ncbi.nlm.nih.gov/pubmed/27259147) contributes to poorer energy and altered neurotransmission, resulting in depression. The SCFAs produced by the gut-friendly bacteria often pass in the systemic circulation and cross the blood-brain barrier through specific transport and enter into the brain. These SCFAs generate neurotransmitters and offers [neuroprotection](https://www.ncbi.nlm.nih.gov/pubmed/24997036) and acting as natural [antidepressants](https://www.ncbi.nlm.nih.gov/pubmed/25818247). **Protein and Vitamin metabolism** Other than carbohydrate metabolism, gut flora also take part in protein and amino acid metabolism through fermentation or putrefaction. However, excessive intake of proteins can result in excessive production of toxic products such as ammonia, putrescine, and phenol, which contribute to [stress and depression](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5682003/). Also, dysbiosis, causing low carbohydrate metabolism and high protein metabolism, find an association with intestinal inflammation leading to [anxiety and depression](https://www.ncbi.nlm.nih.gov/pubmed/30184533). Gut bacteria like Bifidobacterium, which synthesize vitamins like riboflavin, niacin, and folate, occur in a low number in [depressed individuals](https://www.ncbi.nlm.nih.gov/pubmed/28890155). Low folate and thiamine find a strong association with depression and an inverse association with [symptom severity](https://www.ncbi.nlm.nih.gov/pubmed/20841559). **Depression and Dysbiosis** On the other hand, it is reasonable to believe that depression leads to digestive problems and dysbiosis due to unhealthy eating habits or sleep-timings. Decreased ability to digest proteins causes accumulation of residual protein in the colon, aiding the growth of microbes feeding on them. This ultimately leads to elevated putrefaction and gut inflammation. [Several disorders](https://www.ncbi.nlm.nih.gov/pubmed/25964226) linked with dysbiosis, like, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), also find a relation with depression. Overpopulation of pathogenic bacteria causes gut inflammation, damages the gut epithelium and compromises intestinal wall permeability. In severe cases, the bacteria or the intestinal epithelial cells cross the blood-brain barrier, ultimately resulting in anxiety, depression, and related [disorders](https://www.ncbi.nlm.nih.gov/pubmed/21237166). Often activation of the Vagus nerve, controlling digestion, takes responsibility for regulating both dysbiosis and our [mental health](https://www.nature.com/articles/mp201277). Pathogenic microbes in gut flora influence vagal nerve endings distributed in the intestine, causing subsequent pathologic changes in CNS, and propagate depression-like disease symptoms. Additionally, serotonin activates both the vagus nerve and gut motility. **Management** As the gut influences the mind, it is rational to emphasize the role of diet in the management of depression. One way of maintaining the integrity of the gut environment involves the intelligent consumption of Polyunsaturated fatty acids (PUFAs). PUFAs share a relationship with both the gut microbiome and our mood. Interestingly, PUFA balance plays an essential role in the sticking of bacteria on [gut mucus membrane](https://www.ncbi.nlm.nih.gov/pubmed/25498862). Omega-6 PUFA produces molecules that maintain gut mucosal permeability and protect against damage from [inflammation](https://www.ncbi.nlm.nih.gov/pubmed/24473752). _Bifidobacterium,_ capable of degrading PUFAs, are less in number in clinically depressed individuals. Fascinatingly, probiotics containing gut-friendly live microbes, show [antidepressant effects](https://www.ncbi.nlm.nih.gov/pubmed/28483500). _Bifidobacterium longum,_ _Lactobacillus casei,_ and _Lactobacillus rhamnosus_ in probiotics help mend the gut microbial diversity and also elevate anxiety and [depression-like behaviours](https://www.pnas.org/content/108/38/16050). [Faecal microbiota transplant](https://www.ncbi.nlm.nih.gov/pubmed/24762631) is indeed another promising option in correcting depression. **Taken together** Depression and gut flora are entwined. So, to have an idea of the real situation of your gut, it is crucial to have a ‘Gut Microbiome Test’ done. Latest ‘DNA sequencing technology’ and algorithm-based data acquisition aids in identifying the individualized microbiome signature in the stool samples. Based on the results, a treatment plan consisting of a blend of diet, lifestyle habits, and probiotics are articulated. The resultant lifestyle can help you lead a depression free life. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Ulcerative colitis: Multiple issues of Dysbiosis Author: BugSpeaks Published: 2020-02-20 Category: Microbiome and Disease Meta Title: Ulcerative colitis: Multiple issues of Dysbiosis Meta Description:
A common understanding of probable disease-causing mechanisms places diseases as genetic, autoimmune, or/and acquired due to the environment. But w URL: https://www.bugspeaks.com/blog/ulcerative-colitis:-multiple-issues-of-dysbiosis A common understanding of probable disease-causing mechanisms places diseases as genetic, autoimmune, or/and acquired due to the environment. But what if a condition can affect you through any of the three mechanisms. Well, that would be threatening. Such is the story plot of Ulcerative colitis (UC). A life-long illness, which affects the innermost lining of the lower part of the digestive tract, colon, and rectum. Think of ulcerative colitis as a three-headed dragon. It can exhale upon you the fire of ulcerative colitis through heredity, an autoimmune disorder, or [environmental factor](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6167487/). You don't get reduced to ashes but indeed suffer from symptoms like diarrhoea, often accompanied by blood or pus, bowel pain and cramping, rectal bleeding, bloody stool, the urgency to defecate. Consequently, you can lose considerable weight. Generally, the symptoms develop over time, and they differ depending on the location of the inflammation. The type and severity of the symptoms vary from person to person. Though rare, at extreme conditions, UC can be debilitating and can sometimes lead to life-threatening [complications](https://www.ncbi.nlm.nih.gov/pubmed/24060951).  **Gut microbiome: your secret power** Which head of UC dragon gets you depends on how you conduct your life. UC finds close association with the complex ecological system of different candidate microbes residing in our gut. The dominant anaerobic bacteria in the gut form a sort of biofilm over deep-lying tissues. Consequently, they play critical roles in nutrition, permeability, and immune regulation. This group of bacteria, e.g., Bifidobacterium, Bacteroides, and Peptococcus, exist in [symbiotic](https://www.ncbi.nlm.nih.gov/pubmed/23435359) relation with us. Other members of our gut are conditional pathogens, e.g., Enterococcus and Enterobacter. They maintain harmonious relations with the gut of a healthy individual who has ecologically balanced gut flora. But, if you don't take care, this small group of bacteria can become quite detrimental and can damage the gut. The third category contains mostly pathogens, e.g., Proteus and Pseudomonas. In an ecologically balanced gut, these harmful bacteria stay under check. However, hampering of balance due to any external or internal disturbances accentuates the harmful pathogens. They overpopulate and replace the physiological bacteria and resulting in [disease development](https://www.ncbi.nlm.nih.gov/pubmed/23991417). **Keep the Ulcerative colitis dragon tamed** A symbiosis between us and gut microbes promote stability in intestine by inhibiting the colonization of pathogens. Our gut flora under normal circumstances maintains the inner layer of our intestinal tract to keep a relatively low level of inflammation. It helps our gut immune system to continuously screen and eradicate the [detrimental](https://www.ncbi.nlm.nih.gov/pubmed/23435359) microbes. Moreover, the short-chain fatty acids (SCFAs) released by certain healthy gut bacteria, Faecalibacterium prausnitzii, and Roseburia, have several beneficial effects. They are the primary energy source of intestinal cells and also acts as potent modulators of [immune response](https://www.ncbi.nlm.nih.gov/pubmed/23991417). Additionally, they also suppress tumours and act as neuroendocrine modulators of the intestine. However, the most significant function of SCFAs comprises of control over anti-inflammatory genes involved in [gut immunity](https://www.ncbi.nlm.nih.gov/pubmed/24518984). Thus, gut dysbiosis, which results in a loss of diversity of intestinal microbiota, influences UC. In UC, dysbiosis, by approximately 25% as compared with that of healthy controls, results in an invasion of pathogenic bacteria in the intestinal mucosa. Some common gut bacteria, Akkermansia muciniphila, and Roseburia lessen whereas detrimental ones, [Mycobacterium avium paratuberculosis](https://www.ncbi.nlm.nih.gov/pubmed/15984976), adherent-invasive [Escherichia coli](https://www.sciencedirect.com/science/article/abs/pii/S0016508506002757), [Clostridium difficile](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3837256/), [Helicobacter species](https://www.ncbi.nlm.nih.gov/pubmed/19438427)11, [Salmonella species](https://www.ncbi.nlm.nih.gov/pubmed/21383845), [Yersinia](https://www.ncbi.nlm.nih.gov/pubmed/15967332), [Fusobacterium](https://www.ncbi.nlm.nih.gov/pubmed/12477765), [Norovirus](https://www.ncbi.nlm.nih.gov/pubmed/24351661), and [Listeria](https://www.ncbi.nlm.nih.gov/pubmed/12737451)16 increase in UC. These gut damaging bacteria secrete certain toxic elements, which makes the intestinal wall more porous. Furthermore, the enhanced synthesis and secretion of immunosuppressive factors lead to the degeneration of intestinal physiology. All of these ultimately results in immune dysfunction and damaged [intestinal epithelial cells](https://annals.org/aim/article-abstract/718938/new-concepts-pathophysiology-inflammatory-bowel-disease). All this amplifies gut inflammation. So, care for autoimmune reactions and environmental factors by keeping stress at bay and eating healthy fibres is necessary. **Probiotics: Your knight in shining armour?** Gut friendly bacteria in the live state are known as probiotics. Relevant candidates of this group are Lactobacillus, Bifidobacterium, and [Enterococcus](https://www.ncbi.nlm.nih.gov/pubmed/19783474). They play several essential functions in gut physiology. Firstly, they compete with pathogens for adhesion sites and nutrients and replaces the harmful ones, while, they correct the intestinal microbial imbalance. Additionally, they maintain and restore complete intestinal mucosal barrier function. Fourthly, they promote immune tolerance by secretion of anti-inflammatory factors of the intestinal mucosa and thus inhibit pathogenesis. Fifthly, they help to mend the intestinal and total immunity of the body. And lastly, they prevent cell death of intestinal mucosa. Thus, probiotics can effectively induce and maintain at-least temporary remission in [UC patients](https://www.ncbi.nlm.nih.gov/pubmed/16819502). However, some studies associated with probiotics and UC present contradictory results. They have demonstrated that treatment with probiotics amplified bowel movement, resulting in frequent defaecation and diarrhoea like [symptoms](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3901512/#!po=1.21951). Other studies show that probiotics such as Lactobacillus acidophilus LA-5 and Bifidobacterium showed no difference between UC remission patients and the [control group](https://www.ncbi.nlm.nih.gov/pubmed/21453880)20\. Better study design with bigger study group based clinical trials, therefore, remains desirable to establish the exact role of probiotics in UC management. **Faecal Microbiota Transplant: Helping the damsel in distress** Faecal microbiota transplantation (FMT) remains another healthy option for slaying UC. The transplant of faecal bacteria from a healthy donor to a recipient, suffering from UC, can quickly resume the normal function and composition of intestinal microbiota. Don't worry. No one will make you eat the poop. In this process, freshly collected faeces are dissolved and homogenized in saline or water, filtered and transplanted within 6 to 8 hours of collection. After FMT, the microbial diversity in the receivers' gut became very much similar to that of [the donors](https://www.ncbi.nlm.nih.gov/pubmed/21871249). FMT is a simple procedure and can be done with ease. This is also a rather safe mode of treatment. Interestingly, according to some reports, repeated FMT might be better than single transplantation. As, after several years of treatment, there is a chance of microbial reversal. Thus, it is vital to undergo FMT again after several years. However, the number of procedure and gap between two transplantations need to be evaluated depending on the personal diversity in [every case](https://www.ncbi.nlm.nih.gov/pubmed/24060759). The proportion of beneficial bacteria and diversity increases and thus amending dysbiosis. This blend of beneficial bacteria often contains SFCA producing ones, which improve intestinal ecology and permeability of the mucosal wall — thereby lowering the disease intensity. The proportion of Lachnospiraceae, a butyrate-producing bacterium, gets amplified by FMT. It suggests Lachnospiraceae as the vital bacteria in the success of [FMT](https://www.ncbi.nlm.nih.gov/pubmed/24681177). **Adverse reactions of gut flora** Complications of FMT are rare but can be dangerous and life-threatening. The complications include abnormally [low blood pressure](https://www.ncbi.nlm.nih.gov/pubmed/24759832), [norovirus infection](https://www.ncbi.nlm.nih.gov/pubmed/23912408), perforation of the colon, [pneumonia](https://www.ncbi.nlm.nih.gov/pubmed/25982290), [cytomegalovirus](https://www.ncbi.nlm.nih.gov/pubmed/25119613) infection, [bacteraemia](https://www.ncbi.nlm.nih.gov/pubmed/24184170), and others, are just a few among many others. For these reasons, to be on the safe side, long-term follow-up remains essential for patients undergoing FMT. **Gut microbiome test: A personalized armour against UC** UC and gut flora are interlinked. To have an idea of the harbouring microbes in our digestive tract, a 'Gut Microbiome Test' is essential. Cutting-edge' DNA sequencing technology' helps to detect the exclusive range of micro-organisms in patient stool samples. Based on this result, a tailor-made, therapy, and diet are prescribed for every individual. Although a complete treatment of UC remains elusive, highly effective management remains a possibility. With proper treatment, the severity of the disease can be managed up to a large extent. Most people with UC attain long-lasting remission and a hassle-free life with appropriate therapy and lifestyle. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Gluten riddles the gut-land Author: BugSpeaks Published: 2020-02-13 Category: Nutrition Meta Title: Gluten riddles the gut-land Meta Description:
You may eat your delicious grains, but should you or should you not, will be revealed how your gut reacts to something k URL: https://www.bugspeaks.com/blog/gluten-riddles-the-gut-land You may eat your delicious grains, but should you or should you not, will be revealed how your gut reacts to something key within those grains. What is this key thing you must wonder? Well, it is something that remains common in various food grains and constitutes a major food allergen in the western world. If you have guessed it as gluten, then you are right. But yes, the food you eat and drink, affects your health. Such is the curious case of gluten. For some it’s just an everyday family of proteins that nourish the body, while others suffer from bane of gluten intolerance. But gluten isn't a single thing, rather it includes a group of hundreds of diverse proteins. So, yes, it is a party of many proteins primarily comprising of, prolamins and glutelins which may or may not cause you trouble. Gluten occurs with starch and accounts for 75–85% of the total wheat protein. It also occurs in many other grains like barley, rye, spelt, oats and triticale and the products derived from them, like, bread and malts. Gluten acts like glue which makes the food chewy, give it elasticity, and helps food-grains to retain their shape.  #### **Gluten in gut-land** Despite all the delicious gooiness which gluten imparts to food, it can cause some adverse immunological and autoimmune reactions in certain susceptible people. Such a reaction makes up gluten intolerance, which refers to three types of conditions. These [include](https://www.ncbi.nlm.nih.gov/pubmed/27763541) gluten allergy, Celiac disease, and Non-celiac gluten sensitivity. Differentiating between these can rattle one's brains as symptoms may resemble each other. It is essentially a matter of how your gut behaves to the glutens which have dropped down the oesophagus. A gluten allergy caused due to the consumption of gluten, wheat, or related food products may cause symptoms like mild skin rash, respiratory or gastrointestinal reactions or severe life-threatening symptoms like breathing disability followed by anaphylactic shock. Yet, the actual mechanism causing gluten allergies remains a riddle to be solved. Different from an allergic reaction, when gluten triggers an autoimmune reaction outcome the [Celiac disease](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6221985/). In this case, antibodies raised against gluten damages the small finger-like projections in the small intestine called villi. Symptoms include abdominal pain, constipation, stomach pain, vomiting, bloating, nausea, and chronic diarrhoea. Another potential form of gluten intolerance, [Non-celiac gluten sensitivity](https://celiac.org/about-celiac-disease/related-conditions/non-celiac-wheat-gluten-sensitivity/) (NCGS) is neither true gluten allergy nor exact Celiac Disease. It finds association with symptoms in the gut, brain, muscles, skin or elsewhere in response to the consumption of gluten or gluten-containing food products. However, the villi in small intestines remain healthy. The symptoms generally disappear on a gluten-free diet. It is interesting to note that all the conditions find association with gut microbial dysbiosis. **What makes gluten confuse the gut?** Digestion of gluten with our own enzymes in the intestine produces immunogenic polypeptides, which stimulate immunologic cells in our body. These immune-toxic peptides are resistant to further breakdown. Bacteria present in small intestine participate in degradation and metabolism of gluten, which is otherwise is tough to [digest](https://www.ncbi.nlm.nih.gov/pubmed/23244345). The friendly gut bacteria like, Lactobacillus helveticus, Firmicutes and Actinobacteria reduce gluten’s immunogenicity. They produce enzymes that effectively cleave immunogenic polypeptides produced during the initial degradation of [gluten](https://www.ncbi.nlm.nih.gov/pubmed/28526528). But when such friendly microbes are not present as required the immunogenic polypeptides go mad and cause the conditions of gluten intolerance. Experts think dysbiosis as the root cause of gluten intolerance, characterized by an imbalance between beneficial and detrimental bacteria. It has been observed that gluten allergy is associated with reduced numbers of Firmicutes and Bifidobacterium, leading to low levels of [butyrate](https://ngmedicine.com/scfas-part-2-the-benefits-of-butyrate/) in the gut and increased intestinal permeability. Proteolytic enzymes produced by harmful bacteria like Pseudomonas aeruginosa, Bacteroides fragilis and Proteobacteria generate longer peptide products from gluten. These peptides activate [immunologic-cells](https://www.ncbi.nlm.nih.gov/pubmed/23244345). Whereas digestive enzymes produced by beneficial bacteria, Lactobacillus produced non-immunogenic smaller peptides from gluten. Several factors like early-life antibiotic treatment can lead to dysbiosis with an expansion of unfriendly [Proteobacteria.](https://www.ncbi.nlm.nih.gov/pubmed/23244345) **Should the gut-land be devoid of gluten?** As gluten is the culprit behind allergies and other conditions, having a gluten-free diet is the easiest solution to reduce the pro-inflammatory signals via the renewal of gut-microbiome. This offers great relief with improvement in digestion, reduction in digestive tract associated problems, and other symptoms related to [gluten allergy](https://www.ncbi.nlm.nih.gov/pubmed/27373514). Ultimately, this gluten-free lifestyle has an additional benefit, it can help to lose weight. In a gluten-free diet, the healthier options, like quinoa, which doesn't contain gluten replace starches and polysaccharides. However, the gains come at the cost of losses, as the members of gut-land may suffer when starch intake reduces. As in natural conditions, gluten occurs with starch, a gluten deficient diet significantly brings down the starch intake. It is notable to find that, a switch to a diet that is low or free from gluten, also [depletes](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3023594/) the comparative abundance of beneficial butyrate-producing, Clostridiales and [Lachnospiraceae.](https://www.ncbi.nlm.nih.gov/pubmed/25403367) Why? Because one simply takes away what these bacteria feed on. Moreover, this may also provide the opportunistic pathogens such as [Escherichia coli](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3023594/) to proliferate and cause further problems. **Answer lies in Pre- and Pro-biotics** As gluten allergy finds close association with dysbiosis, it is of worth to use pre-biotics or pro-biotics to overpopulate and diversify the gut microbiome. It helps reshape the bacterial community in a more beneficial mode. Such transformed gut flora would help improve gluten tolerance and reduce associated inflammation. Certain healthy gut bacteria like Bifidobacterium longum, Bifidobacterium animalis, and Lactobacillus rhamnosus have been found to diminish the destructive effects of gluten. Prebiotics, like, food loaded with fibre and green leafy vegetables can efficiently replace the pathogens with beneficial ones. Probiotics also help to diminish inflammation and address the fundamental reasons for [gluten allergy](https://www.ncbi.nlm.nih.gov/pubmed/25403367). **Coming out of gluten allergy** A jump out of the dysbiosis of the gut, back to the health is what your gut-land needs. Currently, a complete cure for gluten allergy is not possible and one might have to endure it for long. But there is nothing to lose hope. As gluten allergy and gut microbiome are interconnected, so, to keep it under control, it is crucial to regulate the gut microbiome. Before regulation in an effective manner, testing proves essential, hence the first step should entail a ‘Gut Microbiome Test’. The test uses cutting-edge ‘DNA sequencing technology’ to identify the unique collection of gut flora harbouring in patient stool samples. Then a person-specific tailor-made diet plan is formulated based on the unique gut microbial signature. The signs and symptoms can be kept under control by religiously following a custom well-designed therapeutic plan. Like all other diet-based therapies, it often takes quite some time to get everything resolved. So, patience is the key. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Irritable gut - Irritable you Author: BugSpeaks Published: 2020-02-06 Category: Microbiome and Disease Meta Title: Irritable gut - Irritable you Meta Description:
Like us, our bowel can get irritated too!
In one-word, i URL: https://www.bugspeaks.com/blog/irritable-gut---irritable-you **Like us, our bowel can get irritated too!** In one-word, irritation in our gut may indicate ‘Irritable bowel syndrome’ (IBS). Often associated with chronic, recurrent abdominal discomfort and pain, IBS gets worse after meals and resolves upon defecation. Symptoms include changes in bowel habits, diarrhoea, constipation, or alternating constipation and digestive symptoms, such as dyspepsia, dysphagia, and [nausea](https://www.ncbi.nlm.nih.gov/pubmed/27144627). Sometimes, IBS finds a link with non-gastrointestinal disorders such as chronic pelvic pain, fibromyalgia, chronic fatigue syndrome, anxiety, and even depression. If unaddressed, these comorbidities may hamper your [day-to-day life](https://jamanetwork.com/journals/jama/article-abstract/2174034). A flare-up may last for several days, and then symptoms either improve or resolve completely. Don’t worry. Severe complications are rare in reality. Also, many such comorbidities often find an association with gut dysbiosis. **Why??** A complex assemblage of trillions of micro-organisms including fungi, eukaryotes, bacteria, viruses, and archaea live in our gut. These dynamic and vibrant microbial communities comprise of pro- and anti-inflammatory bacteria and maintain gastrointestinal [homeostasis](https://www.ncbi.nlm.nih.gov/pubmed/24665829). This homeostasis enables microbes to colonize the intestine and perform symbiotic functions persistently. Thus, gut microbes are the critical players in IBS [pathogenesis](https://www.nature.com/articles/nature25973). **How??** Dysbiosis, typically associated with IBS, occurs at a frequency of [73%](https://www.ncbi.nlm.nih.gov/pubmed/27444134). Pre-dominant bacteria contributing to dysbiosis include Bacillus and Ruminococcus gnavus, Shigella or Escherichia, and [Actinobacteria](https://www.ncbi.nlm.nih.gov/pubmed/27444134). Frequently, in IBS, commensals turn pathogenic through the gain of virulence. For example, Enterococcus faecalis turns to non-invasive E. coli, evading the immune system in disease pathogenesis. All this evidence indicates a strong relation between dysbiosis and the severity of [IBS](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4645401/).  **A little off-balance, a lot of imbalance** Certain gut bacteria ferment polysaccharides to produce SCFAs and gasses such as hydrogen (H2) and methane (CH4). Thus, they influence bowel movement and permeability of the intestinal barrier. However, a breach may occur in the intestinal wall due to the influx of inflammatory mediators or pathogens. Consequently, severe inflammation occurs, affecting the gut milieu with alteration of [gut flora](https://www.ncbi.nlm.nih.gov/pubmed/18541210). As expected, a lower abundance of SCFA producing bacteria occurs in IBS, which also maintains lower counts of [methanogens](https://www.ncbi.nlm.nih.gov/pubmed/24751910). Accordingly, microbial metabolism disrupts, resulting in a high production of hydrogen gas, causing flatulence in the [abdomen](https://www.ncbi.nlm.nih.gov/pubmed/20680169). Moreover, IBS subjects relative to healthy controls present overpopulation of Proteobacteria, Veillonella, Firmicutes, Lactobacillus, and [Ruminococcus](https://www.ncbi.nlm.nih.gov/pubmed/22986438). They show a decreased quantity of Bifidobacterium, Faecalibacterium, Erysipelotrichaceae, and [Methanogens](https://www.nature.com/articles/srep12693). Moreover, the severity of IBS finds a positive correlation with a specific intestinal microbiota signature, characterized by low microbial richness, Methanobacteriales absence, and enriched [Bacteroides](https://www.ncbi.nlm.nih.gov/pubmed/24751910). **Psychological Association** A stressful mind stresses your gut, so remains the case with IBS. Considered a stress-sensitive disorder, psychological factors like any abuse, adverse life event, and post-traumatic stress disorder can elicit IBS. How? These conditions are likely to be involved in gut-brain interaction and IBS [pathogenesis](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202343/). IBS and gut dysbiosis find a link with the psychological wellbeing of an individual. A prominent example relates to depression resulting from the activation of colon immunity and the release of certain inflammatory [factors](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5825947/). Another common issue of high stress leads to altered metabolism of serotonin, a neurotransmitter that is responsible for both variations of gut motility and pathogenesis of [IBS](https://theromefoundation.org/wp-content/uploads/functional-gastrointestinal-disorders-history-pathophysiology-clinical-features-and-rome-iv.pdf). **Genetic predisposition** Specific genetic aberrations like a mutation in the [SC5NA gene](https://www.ncbi.nlm.nih.gov/pubmed/24613995) responsible for immune regulation and epithelial barrier function relate to IBS development. Additionally, epigenetic factors also influence [IBS occurrence](https://www.ncbi.nlm.nih.gov/pubmed/26670691). **Therapeutic Options** Probiotics are one of the essential therapeutic options. They play a role in modulating gut inflammation and produce antimicrobial peptides. These products help eliminate pathogenic bacteria and improve the mucosal barrier function easing out the symptoms of IBS. Consistent use of probiotics, the Biofidobacterium or [Lactobacillus](https://www.ncbi.nlm.nih.gov/pubmed/22730468), or a blend of bacteria consisting of Bifidobacterium, Lactobacillus, and Streptococcus help improve the symptoms of [IBS](https://www.ncbi.nlm.nih.gov/pubmed/24979556). However, due to a short lifespan, repeated doses of prebiotics are required. Therefore, prebiotics may serve as a better alternative treatment as they provide substrates that can help in the growth of specific bacteria and hence can alter the [microbiota](https://www.ncbi.nlm.nih.gov/pubmed/18685504). Prebiotics present multipronged benefits. For example, the prebiotic lactulose promotes gut bacteria, water retention in stool, exhibiting laxative effects. Other synthetic prebiotics includes various kinds of oligosaccharides like fructo-oligosaccharides, soybean oligosaccharides, galacto-oligosaccharides, isomalto-oligosaccharides, xylo-oligosaccharides, and others. Accordingly, cereals, fruits, and vegetables are rich in prebiotics. Commensal bacteria in the colon ferment prebiotics to produce SCFAs, which help regulate inflammatory responses, and influence gut homeostasis. Resultantly, dysbiosis gets corrected through the promotion of positive alterations in the gut [microflora](https://www.ncbi.nlm.nih.gov/pubmed/29500265). A step ahead of probiotics and prebiotics, synbiotics, a combination of the two, show higher potency compared to either of [the two](https://www.ncbi.nlm.nih.gov/pubmed/28936910). **A step ahead pro- and prebiotics** Another advancement over probiotics includes paraprobiotic and postbiotic. Paraprobiotics are non-viable or inactivated probiotics, while postbiotics are non-viable soluble factors secreted by live bacteria or released upon [cell lysis](https://www.ncbi.nlm.nih.gov/pubmed/22301383). They benefit the host through their [biological activity](https://www.ncbi.nlm.nih.gov/pubmed/23990841). The advantage of postbiotics and paraprobiotic lies in their safety profile and longer shelf-life while conferring health benefits comparable to those of [probiotics](https://www.ncbi.nlm.nih.gov/pubmed/24336217). Moreover, some poorly/non-absorbed antibiotics with low toxicities, such as rifaximin and neomycin, also prove useful for the treatment of [IBS](https://www.ncbi.nlm.nih.gov/pubmed/23556126). These antibiotics cause remarkable improvements in some instances of IBS known as small intestinal bacterial [overgrowth](https://www.ncbi.nlm.nih.gov/pubmed/27402085). **Dietary Influence** Dietary interventions are helpful can help IBS patients as, in most cases, the consumption of certain foods causes aggravation of [symptoms](https://www.ncbi.nlm.nih.gov/pubmed/26109797). In terms of dietary influences, the main culprits are gluten and short-chain carbohydrates that trigger harmful alterations. A diet that corrects the microbiota dysbiosis could thus help manage IBS [symptoms](https://www.ncbi.nlm.nih.gov/pubmed/26311042). However, meagre consumption of short-chain carbohydrates may reduce the abundance of healthy gut commensal bacteria. Therefore, careful modification or restriction of dietary intake in IBS patients only proves helpful. But, to achieve an effective diet, an individual may need to go through a process of trial and error as IBS has no [universal diet](https://www.sciencedirect.com/science/article/abs/pii/S0924224417302765). **Faecal Microbiota Transplant (FMT)** Interestingly, some other person's excreta can also provide relief from IBS. Although it sounds a bit wicked, the poo treatment, known as Faecal Microbiota Transplant (FMT), can help. FMT involves applying a solution of faecal material from a healthy donor into the gut of a receiver, intending to restore the aberrant microbial [composition](https://www.ncbi.nlm.nih.gov/pubmed/23659729). The technique aims to restore the dysbiosis in the gut. **Finally,** As science continues to disentangle the mysteries of the gut microbiome, the mechanisms to manipulate the gut microbiome for better health continue to widen. With the help of a ‘Gut Microbiome Test,’ it is very easy to obtain an individualized microbiome profile. Through DNA sequencing technologies, experts can now devise personalized tailor-made treatment diet plans, probiotic plans as per the need of the individual patients. There is no cure for IBS. However, as IBS is a chronic condition, so you need to manage it on a long-term basis. It can be kept under control by following the doctor’s advice religiously, maintaining diet, lifestyle, and stress level. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Inflammatory bowel disease: Battle of microbes Author: BugSpeaks Published: 2020-01-30 Category: Microbiome and Disease Meta Title: Inflammatory bowel disease: Battle of microbes Meta Description:
Here comes the superhero to save the day!! Wait, it seems the superhero is suffering from abdominal pain and cramping. Yet the sup URL: https://www.bugspeaks.com/blog/inflammatory-bowel-disease:-battle-of-microbes Here comes the superhero to save the day!! Wait, it seems the superhero is suffering from abdominal pain and cramping. Yet the superhero while pushing through the day, gets the work done. Did you guess the superhero?? No, it is not superman nor spiderman; it is anyone who suffers from Inflammatory bowel disease (IBD). IBD involves chronic inflammation of gut mediated by the immune system and includes Crohn's disease (CD) and ulcerative colitis (UC). A person who suffers from IBD is no less than a superhero pushing through the pain to get the life going. IBD involves the transformation of cells of the inner lining of the gut causing simultaneous destruction and repair of the adjacent tissue. It operates in a person-specific manner in terms of location and behaviour of the disease. So just every superhero has their own super abilities, an individual with IBD presents an alternating pattern of intensification and reduction of the symptoms. Symptoms include abdominal pain, cramping, mild to severe diarrhoea, fever, low appetite, fatigue, and unintended [weight loss](https://www.ncbi.nlm.nih.gov/pubmed/22001864). A severe form of the disease can even lead to life-threatening complications. Though the exact cause remains undiscovered, both genetic predisposition and immune system malfunction may influence IBD. Environmental factors such as diet, lifestyle, antibiotic use, and socioeconomic development exaggerate the [disease](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6167487/).  **Looking for the IBD villain** As IBD involves inflammation of the gut mucosa, it is logical to associate the disease with the dynamic and diverse range of microbiome harbouring in there. The helpful gut bacteria inhibit mucosal inflammation and thus act as a shield against [IBD](https://www.ncbi.nlm.nih.gov/pubmed/23013615). The diversity of beneficial gut microbes declines during the diseased conditions and the equilibrium between commensal and potential pathogen shifts. This villainous takeover of harmful microbes in the gut thus relates to dysbiosis. Moreover, due to the person-specific diversity of gut microbiomes, significant differences occur in equilibrium shifts between [different individuals](https://www.ncbi.nlm.nih.gov/pubmed/28191884). Beneficial bacteria such as _Firmicutes_ and _Bacteroides_ decline_._ Pathogenic _Gammaproteobacteria, Veillonellaceae, Pasteurellacaea, Enterobacteriaceae_, _Fusobacteriaceae_, and adherent-invasive strains of _E. coli_ populate the gut. **Villainous scheme of things in IBD** Amongst the array of microbes affected in IBD comes a set of specific bacteria that ferment indigestible fibres to produce short-chain fatty acids (SCFA). The SCFA producing bacteria, _Faecalibacterium_, _Leuconostocaceae_, _Phascolarctobacterium,_ and _Roseburia_ suffer depletion in the gut. As epithelial cells of colon feed on the energy produced by the [SFCA](https://www.ncbi.nlm.nih.gov/pubmed/23828891), such decline further disrupts the gut energy [metabolism](https://www.ncbi.nlm.nih.gov/pubmed/23013615). Other important function of gut microbiota includes synthesis of some vitamins, enzyme secretion, retention of intestinal mucosal integrity, and educating the gut immune system to prevent the pathogenic attack. Also, they take care of the host by a phenomenon ‘called colonization resistance,’ where they occupy host niches leaving no empty spaces for [invading pathogens](https://www.ncbi.nlm.nih.gov/pubmed/22869189). The gut of healthy individuals also harbours certain beneficial fungus-like, _Saccharomyces, Candida_, and [_Cladosporium_](https://www.ncbi.nlm.nih.gov/pubmed/23799070) and helpful viruses like bacteriophages, _Caudovirales_, and [_Microviridae_](https://www.ncbi.nlm.nih.gov/pubmed/24621522). However, the abundance of some harmful fungi like _Basidiomycota, and Ascomycota_ elevates significantly in [IBD patients](https://www.ncbi.nlm.nih.gov/pubmed/18936492). Another candidate belonging to the healthy gut microbiome is helminths, playing an immune-regulatory role in the gut. Their absence also finds a link with [IBD development](https://www.ncbi.nlm.nih.gov/pubmed/27080105). **What can help the superhero fight IBD villains?** Usual therapies for IBD are [corticosteroids](https://www.ncbi.nlm.nih.gov/pubmed/16236742), [amino-salicylates](https://www.ncbi.nlm.nih.gov/pubmed/15909110), and [immunosuppressive agents](https://www.ncbi.nlm.nih.gov/pubmed/21337668). This symptomatic treatment acts as a quick fix and helps to get rid of complications. Such treatment also comes with adverse side effects, including loss of immune tolerance and [drug resistance](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3084301/). Thus, an alternative effective therapeutic strategy remains the need for the hour. The possibility that gut microbes drive inflammation in IBD inspires several clinical approaches aimed at correcting dysbiosis. These include dietary or microbial interventions like the use of probiotics, antibiotics, defined enteral nutritional therapy (ENT), and faecal microbiota transplantation (FMT). **Probiotics:** Probiotics are a combination of bacteria or yeasts with apparent beneficial health effects that serve to restore [gut microbial balance](https://www.ncbi.nlm.nih.gov/pubmed/23474420). A cocktail of eight live freeze-dried bacterial species has shown to reduce active inflammation and sustain remission in UC, [but not CD](https://www.ncbi.nlm.nih.gov/pubmed/18240278). Friendly bacteria, _Faecalibacterium,_ has shown a protective effect on the intestine by producing SFCA, responsible for barrier-enhancement and immunosuppression. Specific probiotic interventions have shown promise; however, the quest to link clinical improvements of IBD to probiotic-induced microbiota changes continues. Also, fundamental questions regarding the optimal composition of probiotics, timing of administration and durability of the response remain [unanswered](https://www.ncbi.nlm.nih.gov/pubmed/23474420). **Synbiotics:** Synbiotics, a combination of pro- and prebiotics, exert a beneficial effect on host health through working together of two formulations. Pro- and prebiotics in combination, present a novel approach with promising opportunities to evaluate their efficacy and potential [use in IBD](https://www.ncbi.nlm.nih.gov/pubmed/23040451). **Diet:** The diet of an individual exerts a significant effect on the composition of their gut microbiota by altering gut functionality and metabolism at the [genomic level](https://www.ncbi.nlm.nih.gov/pubmed/24503132). One treatment called ENT utilizes the potential of dietary modification. When food is replaced with a nutritionally complete formula in ENT, it can act as a first and foremost line of therapy IBD remission. This treatment results in both clinical improvement and mucosal healing. The idea of providing a fibre-rich diet that can selectively increase the abundance of SCFA-producing microbes remains attractive. Yet, satisfactory outcomes have not been [confirmed](https://www.ncbi.nlm.nih.gov/pubmed/27769810). **Faecal microbiota transplantation:** Antibiotics can ease inflammation, and people often pop them frequently. However, non-specific targeting of gut microbiota with broad-spectrum antibiotics could deplete both beneficial and pathogenic microorganisms. Well, punishing the innocent microbes often comes at its cost, often seriously compromising health. In such a situation, Faecal microbiota transplantation (FMT), or a stool transplant can help. Also helpful with dysbiosis sans antibiotic intake FMT includes transplantation of faecal bacteria from a healthy individual into a recipient. It helps restore the gut microbial homeostasis posing a potential strategy to correct dysbiosis in [IBD](https://www.ncbi.nlm.nih.gov/pubmed/27329806). Overall, data regarding FMT remains scarce. A few points like the safety and durability of this approach, the most effective mode of administration, particularly in immunosuppressed patients, and how to select appropriate donors and recipients should be taken care of. More extensive randomized controlled trials are necessary for a better understanding of the process and to better define the role of FMT in the treatment of IBD. In the future, FMT will be probably be substituted by the use of a defined array of [micro-organisms](https://www.ncbi.nlm.nih.gov/pubmed/23842501). **Winning the battle against IBD** IBD and gut microbiome are closely associated with each other. So, to know the actual scenario of your gut, it is essential to take a ‘Gut Microbiome Test.’ Modern age ‘DNA sequencing technology’ and algorithm-based data acquisition helps to identify the person-specific array of micro-organisms in patient stool samples are. A therapeutic plan consisting of combinations of prebiotics and probiotics are formulated based on the unique microbial population residing in the gut. IBD is not curable. But there is nothing to lose confidence. The one and only way are to manage the signs and symptoms intelligently. Long-term remission is quite possible, with proper guidance and expert advice. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Crohn's disease: No reason to be Hopeless Author: BugSpeaks Published: 2020-01-23 Category: Microbiome and Disease Meta Title: Crohn's disease: No reason to be Hopeless Meta Description:
Our gut harbours diverse flora working like an organ system made up of bacteria, viruses, fungi, and several other microbes. They URL: https://www.bugspeaks.com/blog/crohn's-disease:-no-reason-to-be-hopeless Our gut harbours diverse flora working like an organ system made up of bacteria, viruses, fungi, and several other microbes. They perform a variety of functions, such as training our [immune system](https://www.ncbi.nlm.nih.gov/pubmed/19343057) and suppressing harmful [microorganisms](https://www.ncbi.nlm.nih.gov/pubmed/23748339). But when the balance in the delicate microbial world tips off, dysbiosis occurs, paving the way to an array of health conditions. Crohn’s disease is one such condition wherein the impairment of [gut microbiota](https://www.ncbi.nlm.nih.gov/pubmed/28191884) causes a whole lot of pain to an individual. Down goes the number of healthy bacteria, like, _Firmicutes_ and _Bacteroides_, and pathogenic bacteria, _Gammaproteobacteria, Bacteroidales, Erysipelotrichales_ overpopulate the [gut](https://www.ncbi.nlm.nih.gov/pubmed/23013615). Crohn’s disease includes long term inflammation in bowel with intermittent aggravation and remission. This illness affects different segments of the digestive tract in different people depending on the severity and activity of the disease. Patients suffer from pain in the abdomen, severe diarrhoea, mouth sores, tiredness, malnourishment, and excessive weight loss. Crohn’s disease may either be a fault of inherited genes or the immune system. The exact causation remains unknown. Diet rich in spices, processed food, and stressful lifestyle often worsens the [situation](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6167487/). Quite often, a deeper layer of the gut tissue gets involved, which can pose a [threat to life](https://www.ncbi.nlm.nih.gov/pubmed/26627550).  **The relation between Crohn's and gut microbiome** Some friendly members of gut flora like _Faecalibacterium_, _Phascolarctobacterium,_ and _Roseburia_ ferment dietary fibre to produce short-chain fatty acids (SCFAs). SCFAs like butyrate, propionate, and acetate provide energy and immunity to the [colon](https://www.ncbi.nlm.nih.gov/pubmed/18839424) [epithelium](https://www.ncbi.nlm.nih.gov/pubmed/23842501). Sadly, SCFA producing bacteria decline in the [diseased gut](https://www.ncbi.nlm.nih.gov/pubmed/23842501). Moreover, _Lactobacillus_ and _Bifidobacterium_ populations, which promote protection against gut mucosal inflammation, [also decline](https://www.ncbi.nlm.nih.gov/pubmed/23013615). Like bacteria, numbers of beneficial fungi also fluctuate. For example, _Dioszegia_ and _Candida glabrata_ assume predominance, whereas _Trichosporon_ and _Leptosphaeria_ become a [minority](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4957473/). Once imbalanced, the disturbed microbial diversity further alters the gut environment even during the remission period. For example, the neutral and acidic mucin - a key component of mucus in the gut membrane - secretion remains high during the [remission period](https://www.nature.com/articles/s41598-019-49893-5). It is due to a reduction in bacteria of genera _Oscollospira_ and _Akkermansia_, sulphate-reducing bacteria and _Saccharomyces cerevisiae_ and does not allow relief from inflammation. **Pain with no treatment?** Treating Crohn's diseases is as easy as soft-landing a rover on the moon's lunar surface. No one has done it, yet the efforts and research continue. Antibiotics, your mate in fighting most of the diseases, proves inadequate in Crohn’s disease. The broad-spectrum nontargeted microbiome-destroying approaches do not serve the [purpose](https://www.ncbi.nlm.nih.gov/pubmed/27769810). Increasing knowledge into the promising role of gut microbiota in the plethora of conditions now poses a hopeful avenue for Crohn's patients. The intriguing role of gut microbiota in host immune response ways may hold the key to their function in controlling gut immunity and thus health restoration. **Manipulation of gut microbes: A hope for pain** Aimed at manipulation of the gut microbial population efforts to harness prebiotics, probiotics, and postbiotics to harmonize the gut habitat in Crohn’s disease continues. Prebiotics promote the growth and colonization of gut-friendly organisms. fibre remains as one of the oldest and most frequently used prebiotics. Some beneficial organisms have the unique ability to ferment indigestible fibre to produce SCFA critical to a healthy colon. So, logically, a fibre-rich diet would lower the risk of Crohn’s disease and would have been a line of management in affected people. However, enough evidence lacks this support. Further studies for a better understanding and utilization of fibres in Crohn's disease management [remain warranted](https://www.ncbi.nlm.nih.gov/pubmed/21468064). Knowing the association of Crohn’s disease with dysbiosis, the administration of probiotic strains to help stabilize the gut microbiome seems rational. It may also help redesign the gut habitat in a more advantageous way. This holds the reasonable potential to improve the disease condition. Thus, therapies to restructure microbial communities could be used for improved management of Crohn’s disease. However, more data can help establish the role of probiotic administration as a [therapy](https://www.ncbi.nlm.nih.gov/pubmed/28838410). Also, the negligible clinical achievement with probiotics shows that single or multiple non-target probiotic strain administration may not be helpful. To sustain this treatment, the cocktail of targeted micro-organisms should [be used](https://www.ncbi.nlm.nih.gov/pubmed/28005973). **One man's waste another man's treasure** Another strategy includes the use of postbiotics, the bioactive molecules produced by a microbial community, which maintain the health of the gastrointestinal tract. With the latest cutting-edge technology, identification of vital metabolic pathways and bioactive postbiotics is possible. Administration of postbiotics to patients with Crohn’s disease may help re-establish the full functioning [gut microbial community](https://www.ncbi.nlm.nih.gov/pubmed/28005973). Faecal microbiota transplantation (FMT) is another possible choice of therapy in Crohn’s disease and other diseases with altered microbial compositions. Crohn’s disease has complicated pathogenesis involving host genetics and dysregulation of the host-microbiome crosstalk. The aim of FMT in Crohn’s disease is to cure dysbiosis and re-establish healthy communication between the host immune system and the microbiota. However, due to the complication of the underlying genetic predisposition of the host, success remains limited [in this case](https://www.ncbi.nlm.nih.gov/pubmed/26627550). However, the long-term consequence of FMT as a strategy to treat Crohn’s disease is still not clear. In few years down the lane, as a better approach, FMT will inevitably be replaced with a well-defined blend of [micro-organisms](https://www.ncbi.nlm.nih.gov/pubmed/23842501). **Gut microbiome testing: A personal plan to eliminate Chron's disease** The perturbation or the disturbance of gut flora in Crohn’s disease presents complexity and the mechanisms not yet fully understood. Moreover, it has not been possible to dissect out the dysbiotic changes that, as a consequence, may lead to mucosal inflammation. Severe and chronic inflammation of Crohn’s disease alters intestinal flora. This modification, in turn, leads to atypical host-microbiome interactions, ultimately exacerbating the primary disease. Possibly, through regulation of inflammation or control over the host-microbiome interaction may help disrupt the vicious circle of inflammation and [dysbiosis](https://www.ncbi.nlm.nih.gov/pubmed/26627550). As Crohn’s disease and gut microbiome are entangled, so, to get resolved, it is a prerequisite to taking a ‘Gut Microbiome Test.’ With the help of the latest ‘DNA sequencing technology,’ the exclusive array of micro-organisms in patient stool samples are identified. Then the diet/ therapeutic plan is processed based on the unique microbial population harbouring in the gut. The complete cure of Crohn's disease is so far, not known. But there is hope. Like all chronic illnesses, the only way is to reduce the signs and symptoms significantly. Even long-term remission may be attained through the help of one single therapy or multiple therapies simultaneously or separately. With proper treatment, many people with Crohn's disease can function well and lead an active life. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## C. difficile infection: Managed through Gut Microflora Manipulation Author: BugSpeaks Published: 2020-01-16 Category: Microbiome and Disease Meta Title: C. difficile infection: Managed through Gut Microflora Manipulation Meta Description:
Science now proves the existence of a diverse array of micro-organisms in our gastrointestinal (GI) tract. This variation of gut f URL: https://www.bugspeaks.com/blog/c.-difficile-infection:-managed-through-gut-microflora-manipulation Science now proves the existence of a diverse array of micro-organisms in our gastrointestinal (GI) tract. This variation of gut flora increases through the GI tract from the small intestine to end of the colon. Some bacteria like _Firmicutes_ are predominant in the terminal ileum along with _Streptococcus, Veilonella,_ and _Clostridium_. The colon is highly populated with _Bacteroidetes_ (genus _Bacteroides_) and _Firmicutes_ (genus _Clostridium,_ _Faecalibacterium, Lactobacillus, Ruminococcus_). While only a few _Actinobacteria_ and _Proteobacteria_ reside in the colon. Additionally, some notorious pathogens, like, _Salmonella enterica,_ also comprise 0.1% of total [gut microbiota](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4181834/). _Clostridium difficile_ also belongs to the class of notorious enteric pathogens and resides in the gut of 1-3% of the [healthy population](https://www.ncbi.nlm.nih.gov/pubmed/26038491)_. C._ _difficile_ a spore-forming, gram-positive, and rod-shaped bacterium belongs to the class of enteric pathogens. Through inflammation of the colon, it can cause mild diarrhoea to possibly fatal pseudomembranous colitis and toxic megacolon [disease](https://www.ncbi.nlm.nih.gov/pubmed/24118601). _C._ _difficile_ infection is [prevalent](https://www.ncbi.nlm.nih.gov/pubmed/21762504) in hospitals but may be acquired from the [community](https://www.ncbi.nlm.nih.gov/pubmed/22536816) too. Being an anaerobe, the vegetative form of bacteria hates environments with oxygen. However, bacterial spores survive in the presence of oxygen, harsh temperatures, acidic environment in the [stomach](https://books.google.co.in/books?id=8pKqDwAAQBAJ&printsec=frontcover&dq=Mandell,+Douglas,+and+Bennett%27s+principles+and+practice+of+infectious+diseases.&hl=en&sa=X&ved=0ahUKEwiWrOXAl4bnAhVOWysKHQYhAJQQ6AEIQTAD#v=snippet&q=Clostridium%20difficile%20infection&f=true), and alcohol-containing hand [sanitizers](https://www.ncbi.nlm.nih.gov/pubmed/19715426) as well!  **Journey of** _**C. difficile in the gut**_ These robust _C. difficile_ spores found in soil, faeces, sewage, and other sources thus facilitate disease transmission. _C. difficile_ follows the faecal-oral route of transmission. It spreads either through contact with patients, contaminated objects, or due to [poor hygiene](https://www.ncbi.nlm.nih.gov/pubmed/24814671). Once the spores enter through the mouth, they survive and pass through the stomach to ultimately reach the small intestine. Bile acids and well-known amino acid, glycine, in the small intestine trigger the germination process, leading to [vegetative form](https://www.ncbi.nlm.nih.gov/pubmed/18245298). The vegetative form upon reaching the large intestine flourishes in an oxygen-deficient [environment](https://www.ncbi.nlm.nih.gov/pubmed/25483328). _C difficile_ colonizes on the epithelium of colon and secrete [toxins A and B](https://www.ncbi.nlm.nih.gov/pubmed/17693508). These toxins induce inflammation of the mucosal layer, destroy the epithelial layer and the [intestinal barrier](https://www.ncbi.nlm.nih.gov/pubmed/22237401). **Curious case of** _**C. difficile**_ **and antibiotics** The use of antibiotics also increases the risk of _C. difficile_ [infection](https://www.ncbi.nlm.nih.gov/pubmed/23620467), varying with the type of antibiotic. _C. difficile_ [infection](https://www.ncbi.nlm.nih.gov/pubmed/23478961) finds no association with tetracyclines, while penicillin poses intermediate risk. The fluoroquinolones, third generation cephalosporins, and clindamycin, on the other hand, pose an elevated risk. Using clindamycin poses the maximum risk of C. difficile [infection](https://www.ncbi.nlm.nih.gov/pubmed/24324224). Taking antibiotics even for a few days disturbs the gut flora. While frequent uptake can lead to a profound and [long-lasting damage](https://www.ncbi.nlm.nih.gov/pubmed/18043614). Known as “post-antibiotic dysbiosis”, such impairment includes loss of diversity and change in the metabolism of the microbiota. This altered microbiota exhibits reduced colonization resistance against invading [pathogens](https://www.karger.com/Article/PDF/443360). The number and variety of dominant _Bacteroides_ and _Firmicutes_ drop in association with an overgrowth of [_Enterobacteriaceae_](https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.0060280). Antibiotic consumption reduces the ability of _Bifidobacteriaceae_ to produce acetate_._ Acetate helps protect the epithelial layer of the colon from [infection](https://www.nature.com/articles/nature09646). As the micro-organisms in our gut work hand in hand, the antibiotic usage effects translate far beyond the immediate targets. The loss of one class of bacteria might indirectly hinder the growth and survival of others through mechanisms yet unknown. Consequently, the cumulative outcome of post-antibiotic dysbiosis causes the interruption of [colonization](https://www.ncbi.nlm.nih.gov/pubmed/22595318) resistance against _C. difficile_. **When the acid reduces** _**C. difficile**_ **increase** Another medication which relieves you of heartburn, ulcers and acid problem in stomach favours _C. difficile_ unintentionally. Known as Proton pump inhibitors (PPIs), these medications work by lowering the gastric acid production in the stomach. As the acid reduces, the environment of gut changes, and so does the variety of gut microbiota. Chronic use of PPIs shows a reduction in commensal bacteria, _Bacteroidetes_ and an increase in _Firmicutes_, enhancing the risk of _C. difficile_ [infection](https://www.ncbi.nlm.nih.gov/pubmed/26923470). Other exogenous factors, such as the use of histamine receptors, two blockers, prior hospitalization, and nasogastric tube placement, also find an association with increased risk of _C. difficile_ infection. Some host-related risk factors like the severity of underlying illness, any recent abdominal surgery, aging, and others also influence _C. difficile_ infection. Our gut flora gradually alters with age. Evidence shows that the chance of _C. difficile_ infection increases with approximately 2% for every additional year of age after the [age of 18](https://www.nejm.org/doi/full/10.1056/NEJMoa1012413). In the elderly, the gut microbiota shows the high population of _Bacteroides_ spp. and [_Clostridium_ groups](https://www.ncbi.nlm.nih.gov/pubmed/20571116). However, a noticeable drop in microbial diversity occurs in _C. difficile_ infected patients when compared with a healthy individual of the [same age group](https://www.ncbi.nlm.nih.gov/pubmed/22162545). **Restoring the gut microbiome** Normal and healthy gut microbiota plays a vital role in preventing _C. difficile_ infection by offering colonization resistance against the [pathogen](https://www.ncbi.nlm.nih.gov/pubmed/22595318). Interruption in gut flora composition is profound in patients with recurrent _C. difficile_ infection, particularly those who have received numerous courses of [antibiotics](https://www.ncbi.nlm.nih.gov/pubmed/18199029). Thus, to prevent _C. difficile_ infection, the first and foremost target should be to restore and renew the healthy and beneficial intestinal flora. Another option is to eliminate or at least try to reduce the associated risk factors through the management and manipulation of gut flora back to normal. Thus, to assess the risk of _C. difficile_ infection, all you need to do is to take a ‘Gut Microbiome Test.’ This test uses the cutting-edge DNA sequencing technology and advanced bioinformatics algorithms in a patient faecal sample to map the wide variety of micro-organisms present in the gut. The individual micro-organisms are identified by analysing the massive database of information. Prebiotics, probiotics and custom dietary recommendation prescribed on the basis of results help restore the gut flora back to health. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Gut Microbes: You grow - They Grow Author: BugSpeaks Published: 2020-01-03 Category: Microbiome Meta Title: Gut Microbes: You grow - They Grow Meta Description:
We change with age, our habits, our body, our preferences, and a lot of other changes as we grow. So, when everything in our body URL: https://www.bugspeaks.com/blog/gut-microbes:-you-grow---they-grow We change with age, our habits, our body, our preferences, and a lot of other changes as we grow. So, when everything in our body changes as we age, how can the gut microbes not evolve? Yes, the millions of microbes have been growing and changing with you continuously, helping in healthy existence at every stage of life. The story of gut microbes begins since our inception in the mother’s womb. It is the embryo stage where the microbial colonization of the gut starts. Different ways of in-utero exposure to microbes involve [placenta](https://stm.sciencemag.org/content/6/237/237ra65), [amniotic fluid](https://www.ncbi.nlm.nih.gov/pubmed/22776980), [blood from the umbilical cord](https://www.ncbi.nlm.nih.gov/pubmed/16187156), [semen](https://www.cell.com/servlet/linkout?suffix=e_1_5_1_2_47_2&dbid=4&doi=10.1016/j.cell.2017.11.024&key=10.1016%2Fj.fertnstert.2013.07.1991&cf=pdf&site=fns-site), and [maternal gut microflora](https://www.nature.com/articles/srep23129). The colonization is indicated through similarity in microbial signatures between the amniotic fluid, placenta, and first stool of infant (meconium).  #### **Gut microbiome during birth** As we complete 9-months of gestation in the mother’s womb, the brain releases oxytocin, and childbirth begins. Childbirth marks the second event of microbial transmission. The [nature of delivery](https://www.ncbi.nlm.nih.gov/pubmed/20566857), whether vaginal birth or a caesarean section, determines the type of microflora encountered by the infant. Vaginal delivery exposes the newborn to a range of microbes, such as _Enterobacteriaceae, Lactobacillus, Prevotella,_ which are present throughout the birth canal to the vaginal opening. A caesarean section, on the other hand, limits this interaction for the child and diminishes the diversity of microbial ecology. The microbes encountered during the caesarean section include _Bifidobacteria, Bacteroides, Staphylococcus, Propionibacterium_, and others. They are unique to the maternal epithelial cells’ representative of the interaction with maternal skin alone. #### **Gut microbiome at infancy** Further evolution of colonizing microbes depends on the interactions governed by early life events such as health status and childcare techniques employed by the parents. [Breastfeeding](https://www.ncbi.nlm.nih.gov/pubmed/21300508) is an essential part of ensuring immunity to the baby through antibodies present in breast milk. Besides immunity, it also serves as a critical source of microflora, such as _Bifidobacterium_. Moreover, it has also been found to provide nutrients for the microflora population in the infant’s gut through human milk oligosaccharides (HMOs). New-borns have a gut microflora composed mainly of _Enterobacteriaceae_ and _Staphylococcus_, which later attains _Bifidobacterium_ and _Lactobacillus_. Feeding formula to the infant, on the other hand, can alter the gut microflora at the initial stage of development, resulting in a microbial community with low biomass. #### **Gut microbiome and toddlers** As the infant grows further in age, the shift to [solid food](https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.0050177), dietary supplements, and weaning from breast milk brings in a new wave of microbes such as _Bacteroidetes, Prevotella, Ruminococcus, Clostridium_, and _Veillonell._ The microflora ecology keeps diversifying with age as the number of food sources intensifies, and environmental interactions increase. Notably, the dominance of _Bifidobacteria_ diminishes as solid food is introduced, as the bacteria majorly processes the milk oligosaccharides. Such radical shifts of the microbial taxonomies continue up [to 3 years](https://www.ncbi.nlm.nih.gov/pubmed/20668239) of age as significant alterations in diet occur. #### **Gut microbiome at adolescence** The next stage of radical change in growth and development occurs at puberty. Significant hormonal changes drive the adolescent towards adulthood and sexual maturity with gender-specific modifications. Consequently, the microbial shifts also occur in a gender-specific manner, with women’s gut microflora [maturing earlier](https://www.sciencedaily.com/releases/2019/05/190515093613.htm) than men. Also, with an increase in age, the population of anaerobes increases where that of aerobes and facultative anaerobes decreases. The adolescent microbiome presents higher levels of _Clostridia_ and _Bifidobacteria_ compared to adults and expresses genes related to growth and development. #### **Gut microbiome during adulthood** Following growth and development in adolescence, the adult microbiome transforms to express genes associated with obesity and inflammation. In adulthood, a permanent, stable core community of [colonizers](https://www.ncbi.nlm.nih.gov/pubmed/24956966) exists. It works towards maintaining balance in the body and stable microbial configuration under conditions of duress. As the surface area of gut reaches a peak in the adult, the complexity of gut microbiome increases, which gradually expands to distinct niches in the gut. During pregnancy, the gut microflora also gets altered with an increase in abundance of [_Actinobacteria and Proteobacteria_](https://www.ncbi.nlm.nih.gov/pubmed/22863002)_._ The adult microbiome is susceptible to environmental distresses such as seasonal variation, temperature changes, or exposure to antibiotics. Antibiotics affect the microflora composition; they promote the growth of proteobacteria and reduce the growth of actinobacteria and influence antibiotic resistance. For the later year of life, food and lifestyle have a significant impact on gut microflora[. Adult gut microflora](https://www.ncbi.nlm.nih.gov/pubmed/20571116) consists of _Bacteroides, Firmicutes, Clostridium, Ruminococcus, Eubacterium, Methanobrevibacter_ populations. With advanced age, the microflora ecology consists of _Faecalibacterium_, _Ruminococcus,_ and _Roseburia_, which are mostly obligate and facultative anaerobes. #### **Gut microbiome during older age** Ultimately, in the elder age, the significant variations in the gut microbiome constitution also appear. For example, _Bacteroides, Alistipes,_ and _Parabacteroides_ comprise almost 50% of the core microbiome in the elderly. Also, the maximum fluctuations occur between _Faecalibacterium, Ruminococcus_, and _Clostridium_ IV & XIVa clusters. Even the [living conditions](https://www.nature.com/articles/nature11319) of the elderly have also been indicated to influence the gut microbiome. For example, elderly living in long-term residential care presents a high proportion of Bacteriodetes such as _Coprococcus_ and _Roseburia_. While those living in community dwellings show a high proportion of Firmicutes such as _Coprobacillus, Anaerotruncus, Parabacteroides, Lactonifactor,_ and _Eubacterium._ #### **What does your gut speak to you?** Thus, microflora composition plays a role in the predisposition of an individual to conditions like immune disorders, obesity, allergies, diabetes, neurological disorders, infections, metabolic diseases, and colorectal cancer at different stages of life. In light of the critical role the gut microflora plays in the healthy existence, scientists continue to harbour this knowledge to study the role of the microflora ecology and composition in finding ways to ensure a healthy body. The advancements in DNA sequencing technology allowed the generation of the catalogue of the unique microflora that inherits a host and deduces its role in the health status. And, that’s why we developed [BugSpeaks](https://bugspeaks.com)[®](https://bugspeaks.com). To guide you in healthy ways customized as per your life stage and microflora Bug Speaks test with the help of cutting edge, ‘DNA sequencing technology’ identifies the array of your unique gut microbiome mapped from your faeces sample. The resulting customized results and dietary recommendations allow us to help you propagate healthy gut flora in accordance with your life stage and longevity. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Natural Gut microbiota as an alternative to Vitamin supplements?? Author: BugSpeaks Published: 2019-12-19 Category: Diet and Supplements Meta Title: Natural Gut microbiota as an alternative to Vitamin supplements?? Meta Description:
Our gut serves as a natural habitat for trillions of micro-organisms. To keep us healthy the delicate balance of gut microbiota ne URL: https://www.bugspeaks.com/blog/natural-gut-microbiota-as-an-alternative-to-vitamin-supplements Our gut serves as a natural habitat for trillions of micro-organisms. To keep us healthy the delicate balance of gut microbiota needs harmonious balancing. Gut microbiota, managed by the mucosal barrier, draws a sort of [boundary](https://www.ncbi.nlm.nih.gov/pubmed/24129758) between these tiny tots and us. One of the countless ways these tiny tots help us includes providing several enzymes usually not encoded by our genome. These include the enzymes involved in the breakdown of polysaccharides, polyphenols, and synthesis of most of the [vitamins](https://www.ncbi.nlm.nih.gov/pubmed/28393285). Vitamins or micro-nutrients are biomolecules that act typically as cofactors / co-enzymes for several different enzymatic reactions associated with metabolism and other functions of the [body](https://www.ncbi.nlm.nih.gov/pubmed/27195115). As these little metabolic machineries share unique relation with our micronutrient requirements, an insight into their working take on a high degree of curiosity. #### **Gut microbiome and Vitamin A** Vitamin A or retinoic acid is essential for healthy skin, teeth, skeletal muscle, soft tissue, mucous membrane, central nervous system, and others. Trillion of gut microbes residing in intestinal mucosal lining metabolize Vitamin A obtained through food. One such relationship exists with commensal bacteria of _Clostridia_ class control vitamin A concentration in the gut. In turn, Vitamin A offers higher resistance to colonize the potential [pathogens](https://www.ncbi.nlm.nih.gov/pubmed/30566883) like _Salmonella_ _Typhimurium_. Vitamin A thus plays an important role in homeostatic interactions between the host and the microbiome. #### **Gut microbiome and Vitamin B** Most of the essential vitamins produced by our gut flora typically belong to the B group of vitamins, like, thiamine (B1), riboflavin (B2), niacin/ nicotinic acid (B3), pantothenic acid (B5), pyridoxine (B6), biotin (B7), folic acid (B9) and cobalamin (B12). Folic acid produced by _Lactobacillus_ and [Escherichia coli](https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-018-0534-3) is the most crucial member of the B vitamin family. It is vital for our genetic machinery as it takes a vital role in the biosynthesis of the purines and pyrimidines, the basic building blocks of DNA and RNA. Riboflavin or vitamin B2, yet another vital vitamin, acts as co-enzymes in various vital reactions in cells. While biotin also belonging to B vitamin family helps convert food into energy in mitochondria. A group of useful microbes, _Bacteroidetes_, _Fusobacteria,_ and _Proteobacteria_, harbouring in our intestine own the complete pathway essential for the production of riboflavin and biotin [biosynthesis](https://www.ncbi.nlm.nih.gov/pubmed/31058161). Another member of the group is cobalamin or Vitamin B12. It is a vital micro-nutrient that is responsible for healthy nerve and blood cells. There exists symbiosis or interdependence between us and the cobalamin producing gut micro-flora. For example, _Fusobacteria_, _Pseudomonas,_ and _Klebsiella sp_, present in the gut produce [vitamin B12](https://www.ncbi.nlm.nih.gov/pubmed/25941533). Thus, the microbes aid us by producing cobalamin in the gut. On the other hand, cobalamin protects, preserves, and revives the mucous membrane, the home of the whole [microbial community](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4260394/).  #### **Gut microbiome and vitamin C** Vitamin C has numerous health benefits. So, we find ways to incorporate it into our diet, either by taking supplements or having foods loaded with vitamin C. It boosts our immune system and acts as a critical co-enzyme dealing with oxidative stress and scavenging free [radicals](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5472873/). _Burkholderia_, _Pseudomonas,_ and _Erwinia_ reside in the gut of normal healthy individuals. In the case of people suffering from Crohn’s disease, an **inflammatory bowel disease, these bacteria gain the capability of producing vitamin C, helping reduce inflammation.** However, these bacteria in normal conditions are incapable of producing [vitamin](https://www.nature.com/articles/s41575-018-0044-3). #### **Gut microbiome and vitamin D** Everyone knows about the relationship between sunlight and Vitamin D., But a recent Canadian study sheds light on the plausible association between microbes, vitamin D, and sunlight. [Findings](https://www.frontiersin.org/articles/10.3389/fmicb.2019.02410/full) suggest that exposure to sunlight, more precisely, UVB light can change the human gut microbiome. This alteration is more evident in vitamin D deficient people. Sunlight/ UVB light through the synthesis of vitamin D helps maintain healthy bones and teeth. This vitamin also alters gut flora and helps to overpopulate the number of healthy bacteria present in our digestive tract. Enrichment of the good bacterial family of _Lachnospiracea_e and _Firmicutes_ and the decline of bad _Bacteroidetes_ in the gut following exposure to sunlight has been observed. Thus, _Lachnospiracea_es and _Firmicutes_ group of bacteria often present themselves in the gut of healthy individuals. Whereas, the people suffering from various inflammatory diseases lack the previously mentioned microbes in their gut. #### **Gut microbiome and vitamin E** Lack of Vitamin D renders the body prone to inflammatory diseases, while a lack of Vitamin E or ∞-tocopherol can impair antioxidant activities in our body that helps us get rid of the free radicals. These free radicals are otherwise detrimental to our health. However, the role of such a critical vitamin on gut microbiota composition and vice versa is still under [characterized](https://www.nature.com/articles/s41598-019-46193-w). Korean researchers showed an association between an altered gut microbiome composition and a low intake of vitamin E in [mice](https://www.ncbi.nlm.nih.gov/pubmed/31298050). Hopefully, more studies in the future will help delineate the effect of vitamin E on the intestinal flora. #### **Gut microbiome and vitamin K** To help you control blood loss upon injury Vitamin K comes to rescue. It is an essential factor for blood coagulation and also helps in the calcification of [bones](https://lpi.oregonstate.edu/mic/vitamins/vitamin-K). Vitamin K comprises of two structurally similar isoforms: vitamin K1 and vitamin K2. We get vitamin K1 through the green leafy vegetables, which we eat. However, for vitamin K2, we are dependent on our gut bacterial community. Our intestinal flora converts vitamin [K1 to K2](https://www.ncbi.nlm.nih.gov/pubmed/22940212). [Escherichia coli](https://www.ncbi.nlm.nih.gov/pubmed/22502809) and [Lactobacilli](https://www.ncbi.nlm.nih.gov/pubmed/22502809) present in our gut can also bio-synthesizes vitamin K2. However, vitamin K1 is not produced in our gut. All the evidentiary material glorifies the intricate relationship between microbes and vitamins. The composition of the human gut microbiota, as established during the early years after birth, gets altered over time. However, under the influence of lifestyle factors and usage of drugs and antibiotics, the composition of gut bacterial species could go down the wrong road. Such changes may alter biosynthesis of vitamins, which are essential for our metabolism, [adversely](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4315779/). **To conclude,** So, for an ample supply of the essential vitamins vital for a healthy life, we need healthy gut flora. If a vitamin deficiency is caused by various reasons, to deal with it in a better way, a ‘Gut Microbiome Test’ can always help. In this process, with the help of cutting edge ‘DNA sequencing technology,’ the array of your unique gut microbiome will be identified and mapped from your faeces sample. Based on this, a tailor-made diet plan will be recommended to you. As it takes a while to change your gut microbiome, you must be patient to get good results. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Sound gut for Sound Sleep Author: BugSpeaks Published: 2019-12-05 Category: Microbiome and Lifestyle Meta Title: Sound gut for Sound Sleep Meta Description:
Almost all of us have experienced sleeplessness some time in our life. Occasional sleep disruption is quite reasonable. However, i URL: https://www.bugspeaks.com/blog/sound-gut-for-sound-sleep Almost all of us have experienced sleeplessness some time in our life. Occasional sleep disruption is quite reasonable. However, it’s reasonably problematic to have a sleep problem regularly. Lack of good night sleep daily can restrict your everyday activities. It may seriously affect your quality of life through an adverse impact on mood, work efficiency, stress handling, and others. According to [Mayo Clinic](https://www.mayoclinic.org/diseases-conditions/insomnia/symptoms-causes/syc-20355167), ‘A sleep disorder or insomnia is a condition that frequently impacts your ability to get enough good quality sleep’. It can arise from all sorts of stresses in your life, disrupted sleep-wake cycles, including jet lag, frequent change in work shifts, and poor lifestyle, like having a heavy meal or alcohol in the late evening or watching television till night. Insomnia often finds an association with medical conditions like **mental health disorders,** or [use of medications](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4634348/) like antidepressants, asthma or blood pressure, or products containing caffeine. You would be quite surprised to know that disrupted sleep is often associated with poor gut microbial health! **Sleeping for a healthy gut microbiome** Trillions of micro-organisms inhabit your gut remains a known fact. They [outnumber](https://www.ncbi.nlm.nih.gov/pubmed/16497592/) the total of the cells in your body by at-least ten times. These micro-organisms form an ecosystem of their own and known as the microbiome. The various kinds of intestinal micro-organisms display a dynamic equilibrium in a healthy individual. This subtle balance is vital for health, and a slight imbalance increases the [host’s](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3027896/) [vulnerability](https://www.nature.com/articles/nature08821) to [disease](https://www.physiology.org/doi/full/10.1152/physrev.00045.2009). It's quite apparent that diet would have the most significant influence on your gastro-intestinal tract and the micro-organisms residing in there. However, a recent review conducted by [Dr. James F. Cheeseman](https://unidirectory.auckland.ac.nz/profile/j-cheeseman) from the University of Auckland, New Zealand, established that not only the food we consume but also the sleep patterns are also connected to our [gut microbiome](https://www.ncbi.nlm.nih.gov/pubmed/30709031). The researchers have observed that [gut microbiome](https://www.nature.com/articles/nature06244) plays a significant role in [circadian clock](https://www.ncbi.nlm.nih.gov/pubmed/25891358) function along with other phenotypic characteristics, like, immunity, [metabolism](https://www.ncbi.nlm.nih.gov/pubmed/20040631), and others. The circadian rhythm is our inner clock, which controls our body’s energy disbursement, hunger, and snooze. We usually get about seven hours of sound sleep every night. In the morning, when we wake up, our body warms up to carry out daily chores. To run our body, we need energy, and energy comes from the food we eat during the day. At night, our body needs rest to rewind, so we fast and go to [sleep](https://www.ncbi.nlm.nih.gov/pubmed/30568608). **Gut microbiome resonates with the bodily rhythm** The scientific world now accepts the robust connection between sleep and intestinal wellbeing. A good quality night sleep allows more flourishing and better functioning gut microbiome and [vice versa](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1395357/). Researchers report the reflection of our body’s underlying rhythmicity in the intestinal flora too. For example, _Firmicutes sp_. remains overpopulated during the daytime when we have frequent meals. While, the number of some gut flora such as _Bacteroidetes_ _sp._, _Proteobacteria sp.,_ and _Verrucomicrobia sp_. increases during the night when we sleep and fast. Generally, the microbes which grow on food derived fibres grow at night, as what we consume during the day reaches the colon by [night time](https://www.ncbi.nlm.nih.gov/pubmed/24882755). Gut flora follows the rhythm by secreting specific molecules at certain times of the day. At night, secretion of factors responsible for energy metabolism, DNA repair, and proliferation occurs. During day-time, flora [harbouring in the gut](https://www.ncbi.nlm.nih.gov/pubmed/31123355) releases molecules essential for their colonization. As in circadian rhythm, we fast and sleep during the night. It is advantageous to maintain a healthy gut microbiome. During fasting the _Bifidobacteria sp._, _Ruminococcus bromii_ and _Clostridium chartatabidum_, produce [short-chain fatty acids](https://www.gutmicrobiotaforhealth.com/en/short-chain-fatty-acids/), including, butyrate, and propionate. Butyrate protects our gut and regulates glucose levels by a complex network of metabolites and various [hormones](https://www.ncbi.nlm.nih.gov/pubmed/29903615). Propionate plays a crucial role in fat metabolism in [liver](https://www.ncbi.nlm.nih.gov/pubmed/29903615). The scenario is entirely different at mealtimes, where the release of factors helpful for lipid and protein adsorption occurs.  **Gut microbiome, sleep, and hormones** Neurotransmitters like serotonin and GABA secreted by brain control our [sleep-wake cycle](https://www.ncbi.nlm.nih.gov/pubmed/22025877). Astoundingly, certain intestinal bacteria including, _Turicibacter sanguinis_ and _Clostridia_ _sp.,_ release specific signalling molecules that trigger the production of [serotonin](https://www.nature.com/articles/s41564-019-0540-4). By modulating serotonin levels, the gut microbiome can interfere or improve our sleep pattern. Melatonin, the sleep hormone generated from serotonin, helps to improve our [sleep](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6445894/). Interesting enough, studies have shown that ingesting probiotics may help increase melatonin secretion and sleeping quality, opening an avenue for the future [use of probiotics for sleep regulation](https://www.gutmicrobiotaforhealth.com/en/new-data-link-gut-bacteria-melatonin-circadian-clock/), gut health, and overall well-being16. Alteration in the gut flora often can induce inflammation [in the body](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4056765/), which may lead to [sleep deprivation](https://www.ncbi.nlm.nih.gov/pubmed/24409051) as well. It leads to a vicious cycle of sleep deprivation, which can exert an adverse influence on the well-being of gut microbiome and _vice-versa_. Thus, ignoring sleep problems and disorders can end up badly for your physical health. Stress and anxiety due to sleep deprivation can lead to accidents, poor performance at work, and memory-related issues. Poor gut health due to lack of sleep can also lead to excessive weight gain, which plays a central role in many life-threatening diseases. **Testing little gut microbes for healthy sleep** By now, you may have a clear picture in your mind showing the entangled relation of the health of your gut microbiome and sleep. So, to resolve sleep disorders, common sleeping pills or CPAP machines will not help. To cure it of its root, all you need is to get a ‘Gut Microbiome Test’ done. ‘Gut Microbiome Test’ uses sophisticated ‘DNA sequencing technology’ inpatient stool samples to identify the exclusive collection of micro-organisms existing in your gut. Then this data is used to provide a diet plan exclusively for you, comprising of gut-friendly, plant-based, and fibre-rich food with no added sugar. You will also be advised to have a lifestyle supporting your body clock, like regularly having a meal at least a couple of hours before going to bed, and strictly restricting snacks/ light meals before going to bed. Therefore, this plan aimed to rectify your sleep issues by improving your gut microbiome. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## From Gut to Joints: Microbiome & Arthritis Author: BugSpeaks Published: 2019-12-02 Category: Microbiome and Disease Meta Title: From Gut to Joints: Microbiome & Arthritis Meta Description:
Arthritis is swelling and tenderness of one or more joints in your body. This disease is widespread, yet, not well understood. Fro URL: https://www.bugspeaks.com/blog/from-gut-to-joints:-microbiome-&-arthritis Arthritis is swelling and tenderness of one or more joints in your body. This disease is widespread, yet, not well understood. From the 100 different types of arthritis, osteoarthritis and rheumatoid arthritis represent the most common ones. Regardless of the type, symptoms are similar in all the cases, which include joint pain and stiffness. The symptoms typically worsen with age. However, people of all ages, sexes, and races have arthritis, with more prevalence in women. Being the leading cause of disability in the world, arthritis, often compromises quality of life. Moreover, upon affecting weight-bearing joints, arthritis restricts one from walking comfortably or even sitting up straight. Per the treatment a doctor often advises medications, physical therapy, weight loss and exercise. But a diet alteration to transform your gut microbiome forms a rare recommendation. However, experts at world-renowned mainstream institutions nowadays acknowledge the role of the gut microbiome in certain types of arthritis, autoimmune arthritis. Autoimmune arthritis further includes rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. **Few pieces of evidence:** Scientists have observed the digestive tracts of people with rheumatoid arthritis to be overpopulated with potentially disease-causing bacteria. Microbes like [Prevotella copri](https://ard.bmj.com/content/78/5/590), [Collinsella sp.](https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-016-0299-7), and [Eggerthella lenta](https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-016-0299-7) occur in high numbers while the number of healthy bacteria, like, [Bifidobacterium](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4973033/), the [Porphyromonas-Prevotella](https://www.ncbi.nlm.nih.gov/pubmed/18528968) group of bacteria, [Bacteroides fragilis](https://www.ncbi.nlm.nih.gov/pubmed/18528968) subgroup, [Eubacterium rectale](https://www.ncbi.nlm.nih.gov/pubmed/18528968), and [Clostridium coccoides](https://www.ncbi.nlm.nih.gov/pubmed/18528968) group is relatively low. Also, people carrying the [HLA B27 gene](https://www.ncbi.nlm.nih.gov/pubmed/29287307) show a higher risk of developing autoimmune arthritis. This gene presents an association with changes in the gut microbiome. Thus, it presents a link between gut flora and rheumatoid arthritis and other forms of autoimmune arthritis. **Gut microbiome and autoimmune arthritis** Our gastrointestinal tract is home to a large and complex community of [micro-organisms](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3728647/). This gut microbiota keeps our body fit in many ways. These include healthy digestion, production of nutrients, detoxification, fat metabolism and storage, angiogenesis regulation, protection against pathogens, and regulation of the [immune system](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3728647/). However, overtime, under the influence of altered food habits, lifestyle, and medication (antibiotics and steroids) intake it undergoes modification. These alterations frequently lead to the imbalance of the intestinal microbiome. An imbalance in the gut microbiome can lead to chronic inflammatory and autoimmune diseases, such as arthritis.  **Leaky Gut Syndrome and Arthritis** As per researchers and clinicians ‘Leaky Gut Syndrome,’ characterized with increased intestinal permeability also plays a role in arthritis. The syndrome results from the damage to the small intestine wall. When healthy, the small intestine only allows useful nutrients to pass across and enter the bloodstream. However, upon damage, the intestine permeability stands compromised and larger molecules like half-digested fats, proteins, starches, and even bacteria reach the blood. These microbes are often gram-negative gut bacteria whose outer membranes contain molecules called lipopolysaccharides. The body recognizes lipopolysaccharides and partially digested food products as foreign substances, and they trigger an immune response. The antibodies thus produced, travel through the bloodstream and create an inflammatory response in other parts of the body, including joints, leading to [arthritic pain](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5610356/) and [swelling](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5440529/). Though the matter is controversial, yet there is evidence that shows that healing a leaky gut with a strict diet and nutritional supplements can help to control arthritis. **Inflammation and Arthritis** Inflammation is a common feature in almost all types of arthritis such as rheumatoid arthritis, gout, ankylosing spondylitis, psoriatic arthritis, and pseudogout. Even osteoarthritis—which, lacks an autoimmune response— may present with inflammation. A healthy gut microbiota find implication in inflammation control by influencing the differentiation of inflammatory cell types, and production of regulatory molecules, [like cytokines](https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.118.313234). **A healthy gut means a healthy body** These days correlation between intestinal health and the gut microbiome remains well-understood. So, the best way to deal with arthritis is to maintain a healthy diet to have a healthy gut flora. Diet alteration stands as a great way to achieve this but offers temporary and quick shift in the gut microbiome. To instil a permanent shift toward healthy gut flora a long-term commitment to a healthy diet full of leafy vegetables and plant-based whole foods remains essential. A fibre-rich diet helps beneficial gut flora to flourish. They produce short-chain fatty acids from fibre present in foods. These short-chain fatty acids contribute to the wellbeing of the intestinal lining and may prevent [disease](https://www.ncbi.nlm.nih.gov/pubmed/16633129). Moreover, short-chain fatty acids help curb glycaemic response and satiety, promote weight loss, enhance mineral absorption, and reduce systemic [inflammation.](https://www.ncbi.nlm.nih.gov/pubmed/21840809) All of these are important for the management of arthritis. These changes can lead to a better functioning immune system, reduce inflammation, and lower arthritis-related [pain](https://www.ncbi.nlm.nih.gov/pubmed/23880135). On the other hand, the bacteria thriving on a high-protein, low-fibre diet produces ammonia and other compounds, having potentially harmful effects. Therefore, a healthy, balanced gut microbiome fosters healthy intestines, and vice versa, both of which play a crucial role in a person’s overall health. **Other factors** In addition to diet, other factors, including heredity, stress, and the environment also influence the gut microbiome. For this reason, regular exercise, meditation, and reduction in exposure to toxins must accompany gut rehabilitation through a healthy diet. Physical exercise also alters the composition and functional capacity of the gut microbiota, independent of diet. However, exercise-induced alterations of gut microbiota may depend on obesity status, exercise modality, and [exercise intensity](https://www.ncbi.nlm.nih.gov/pubmed/30883471). **Get ‘Gut Microbiome Test’** As we all have a unique set of gut-microbiota, the dietary recommendations must be tailor-made. So, if you want to fix your arthritis before it gets worse, take a ‘Gut Microbiome Test.’ This test uses cutting edge DNA sequencing technology in patient stool samples to map and identify the unique array of micro-organisms present in the gut. Then this data is used to provide a customized dietary recommendation consisting of probiotics and prebiotics. This diet is targeted at the improvement of arthritis. As it may take quite a few months to get your intestinal flora changed, you are advised to follow the diet plan religiously for several months before deciding whether it works or not. Patience is the key to succeed through gradual progress. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Resolving constipation at microbial level Author: BugSpeaks Published: 2019-11-21 Category: Microbiome and Disease Meta Title: Resolving constipation at microbial level Meta Description:
You will surely be surprised to know that your gut, a big long muscle, more than six meters long, is home to trillions o URL: https://www.bugspeaks.com/blog/resolving-constipation-at-microbial-level You will surely be surprised to know that your gut, a big long muscle, more than six meters long, is home to trillions of microorganisms such as bacteria, archaea, viruses, fungi, metazoa, and protozoa. Each of the microbes has more than [1000 different species](https://www.ncbi.nlm.nih.gov/pubmed/27478747) in there. These communities of gut microbes work in harmony with the human body and evolve continually. The microbes come together to work in cooperation and competition and maintain balance in [microbiome](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4727996/). Nevertheless, some of them can be harmful too. This vast array of gut-harbouring micro-organisms is exposed to the food you eat on a day-to-day basis. Thus, the food essentially has a far-reaching influence on the gut microbiome harmony and functioning4. Increased use of antibiotics, drugs, probiotics, and dietary changes disrupts the delicate balance of the gut microbiome. This imbalance further enhances vulnerability to infections, gastrointestinal tract [inflammation](https://www.ncbi.nlm.nih.gov/pubmed/26460205) and chronic illnesses ranging from metabolic diseases to [gastrointestinal disorders](https://www.ncbi.nlm.nih.gov/pubmed/31315227).  #### **Microbial issues of constipated gut** The discussion on gastrointestinal disorders evokes the most common issue of constipation. What you eat reaches the large intestine in a couple of hours and exits body the next morning. However, the process can, [at times, take up to a week,](http://www.aboutconstipation.org/site/what-is-constipation/normal-function) and the result is a constipated stomach. It’s a sort of little awkward topic that no one wants to discuss openly, yet many suffer. A gut health survey conducted by Abbott Healthcare in 2018 showed that 22% of the country's adults suffer from constipation. Interestingly constipation shows higher prevalence in metro cities believed to be mostly due to urban diet and [lifestyle](https://www.ncbi.nlm.nih.gov/pubmed/27478747). Additionally, one-fourth of constipated individuals suffer from severe pain. Constipation is clinically defined by difficult, infrequent, or inadequate [bowel movements](https://www.gastrojournal.org/article/S0016-5085\(16\)00222-5/abstract). It is one of the most common gastrointestinal diseases, which may result from a variety of reasons. Continued research shows altered gut flora as one of the primary causes of [constipation](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6379309/). For example, the number of certain bacterial species like Bifidobacteria, Lactobacilli, and Bacteroides decrease in constipated adults. Lower levels of Bifidobacteria and high level of Bacteroidetes cause both functional constipation and constipation type irritable bowel syndrome. Also, the numbers of potentially pathogenic microorganisms like Pseudomonas aeruginosa and Campylobacter jejuni increase in people suffering from [constipation](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3145058/). Such alteration in microbiome populations disrupts several metabolic processes causing constipation. #### **Altered gut flora and constipation** **_Metabolism_** Studies show that gut microbiota helps metabolize substances using special enzymes which are not available in humans. Thereby, they help metabolize cholesterol, bile acid, and hormones. Moreover, gut microbes also produce short-chain fatty acids that prove beneficial in numerous ways. For example, bacteria produce short-chain fatty acids like butyrate, acetate, and propionate to carbohydrates in the absence of [oxygen](https://www.hindawi.com/journals/ecam/2019/9372563/). A high concentration of butyrate inhibits mucin secretion and stimulates water and electrolyte absorption in the large intestine. The absence of mucin and water retention reduces the stool volume and increases hardness leading to [constipation](https://www.ncbi.nlm.nih.gov/pubmed/24967088). Correspondingly, research findings support an increase in butyrate-producing genera in constipated [patients](https://www.ncbi.nlm.nih.gov/pubmed/28393285). **_Immunity_** Gut microbiome also concerns immunity as a huge proportion of the immune system finds an association with gut health. For example, Clostridial species, Bacteroidetes species, and Candidatus svagella are found to disrupt colonic immunity. Colonic immunity ultimately causes the development of colonic motility disorder and chronic constipation. They act through the secretion of anti-inflammatory molecules and through function modulation of immune cells. These changes further impact the motility and secretory functions of intestine by altering the amount of physiologically active substances and gut’s metabolic [environment](https://www.ncbi.nlm.nih.gov/pubmed/27478747). Thus, intestinal flora modulates gut mobility by altering bacterial metabolites (such as SCFAs) or bacterial cell components (such as lipopolysaccharide) or through interactions between bacterial cells and the host immune system. Consequently, showing a crucial role in the pathology of constipation. #### **Gut microbes and gastrointestinal movements** Another microbe, Methanobrevibacter smithii, in concert with gut bacteria produces methane from dietary carbohydrates. The methane produced slows down gastric transit. Research supports its role in constipation as these micro-organisms remain abundant in constipated [individuals](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4769647/). Some indirect evidence also shows that gut microbiota regulates endocrine cells to secrete hormones like somatostatin and Neuropeptide Y, which finally modulate the gut motility and constipation1. Further, saturated long-chain fatty acid (SLCFA) generated by gut bacteria enhances colonic contraction and increases stool frequency in [rat models](https://www.ncbi.nlm.nih.gov/pubmed/11171619). Thus, low abundance or absence of bacteria producing SLCFA may play role constipation. Moreover, the bacterial endotoxin lipopolysaccharide influences intestinal motility by delaying gastric emptying and inducing sphincteric [dysfunction](https://www.ncbi.nlm.nih.gov/pubmed/29903041). Hence over-population of bacteria producing lipopolysaccharide in the gut can be a reason for developing constipation. For decades, people with constipation receive advise to change their diets, consume a fibre-rich diet with plenty of probiotics, and indulge in regular physical exercise. However, one size does not fit all, nor do these recommendations. The bacterial community within the stomach and intestines in each and every person remains unique. Thus, a tailor-made plan is needed for every constipated gut. So, if you really want to build a diet that promotes your gut health, the ‘Gut Microbiome Test’ may be your saviour. Using DNA sequencing technology and advance bio-informatics algorithm, the test maps the array of micro-organisms present in your gut. With the help of an advanced artificial intelligence algorithm based on the nutrient database and disease susceptibility index, the BugSpeaks test provides a customized dietary recommendation. This diet targets the root cause of constipation and helps the bowels move freely. --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Best Podcasts on Microbiome Author: BugSpeaks Published: 2019-09-23 Category: Microbiome Meta Title: Best Podcasts on Microbiome Meta Description:
We are living in the "golden-age-of-podcasts”, with an URL: https://www.bugspeaks.com/blog/best-podcasts-on-microbiome We are living in the "[golden-age-of-podcasts](https://chartable.com/blog/golden-age-of-podcasts)”, with an increasing of people listening to podcasts daily or at least once a week. There has been a huge content explosion in this audio consumption format, with ~600 podcasts being started every day. Podcasts have opened different ways of consuming news, articles and even whole books, along with building habits, around walking, jogging and even daily commute to your workplace. Another field that has witnessed a relatively similar rise in its adoption, is the gut microbiome testing in context of one's health and wellness. Leucine Rich Bio's BugSpeaks®, is India's first and only gut microbiome-based test, which is catering to a wide range of consumers committed to improve their overall health and wellness. Merging the best of both worlds, here's a list of Podcasts that will give u a head start on both ends. If you are already into podcasts, and you are one of those who have or who are willing to adopt a healthy lifestyle, then you can start understanding what and how does one's gut microbiome can aid you in achieving your health goals, through these podcasts. On the other hand, you are already into a healthy lifestyle, and wants to keep constant tab on the most updated information about being healthy, especially through gut microbiome, then you can build a habit of listening these podcasts, which will have all the information you need at our finger tips. So, here are some of the most consumed podcasts involving gut health, microbiome and overall wellness.  The Microbiome Report Bio The microbiome. It dictates so much of how we move through the world – from how we digest our food to the mates we choose as we spin around the globe. On this show, we're investigating how the things we do everyday impacts the bugs of our bodies. The Microbiome Report is powered by BIOHM Health. [Apple Podcasts](https://podcasts.apple.com/us/podcast/the-microbiome-report/id1443154886) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly90aGVtaWNyb2Jpb21lcmVwb3J0LmxpYnN5bi5jb20vcnNz) [PlayerFM](https://player.fm/series/the-microbiome-report) The Perfect Stool Understanding and Healing the Gut Microbiome Lindsey Parsons Bio Hear host Lindsey Parsons interview functional and integrative medicine professionals, patients and scientists about the gut microbiome, the current state of research and how you can apply it to your life. Hear patients talk about their experiences with fecal microbiota transplantation (FMT) both in clinics and do-it-yourself and how it impacted their gut issues. Learn about the gut microbiome's influence not just on digestive health, but on cardiovascular health, mental health, autoimmune diseases, skin conditions and overweight/obesity. [Apple Podcasts](https://podcasts.apple.com/us/podcast/perfect-stool-understanding-healing-gut-microbiome/id1444156616) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly90aGVwZXJmZWN0c3Rvb2wubGlic3luLmNvbS9yc3M) [PlayerFM](https://player.fm/series/the-perfect-stool-understanding-and-healing-the-gut-microbiome)   BacterioFiles Bio The podcast for microbe lovers: reporting on exciting news about bacteria, archaea, and sometimes even eukaryotic microbes and viruses. [Apple Podcasts](https://podcasts.apple.com/us/podcast/bacteriofiles/id352470437) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cDovL2JhY3RlcmlvZmlsZXMubWljcm9iZXdvcmxkLmxpYnN5bnByby5jb20vcnNz) [PlayerFM](https://player.fm/series/series-1567470) Metagenics Clinical Podcast Bio Welcome to the Metagenics Clinical Podcast where Natural Healthcare Practitioners can hear innovative, cutting-edge information from leading experts from around the world. Join us while we explore the latest evidence in Natural Health, challenge and debunk industry myths and offer practical, tangible, clinical tools which will transform your practice [Apple Podcasts](https://podcasts.apple.com/au/podcast/metagenics-clinical-podcast/id1177865679) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly9mZWVkcy5maXJlc2lkZS5mbS9tZXRhZ2VuaWNzY2xpbmljYWxwb2RjYXN0L3Jzcw) [PlayerFM](https://player.fm/series/metagenics-clinical-podcast)   Microbe Talk Bio Microbe Talk is a podcast from the Microbiology Society, interviewing researchers about bacteria, viruses and parasites. We are the largest microbiology society in Europe, covering all aspects of microbial science. [Apple Podcasts](https://podcasts.apple.com/us/podcast/microbe-talk/id505999112) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly9taWNyb2JldGFsay5wb2RvbWF0aWMuY29tL3JzczIueG1s) [PlayerFM](https://player.fm/series/microbe-talk) The Doctor's Kitchen Podcast Bio Welcome to The Doctor's Kitchen Podcast with Dr Rupy Aujla. Covering a range of topics from the principles of healthy eating to how to prevent and treat illness, Dr Rupy and his panel of experts draw on the latest research to give you actionable tips to help supercharge your health.What you choose to put on your plate is one of the most important health interventions anyone can make. Dr Rupy's cooksbook, The Doctor's Kitchen and his latest, Eat To Beat Illness, are out now all good bookshops and ebook. [Apple Podcasts](https://podcasts.apple.com/gb/podcast/the-doctors-kitchen-podcast/id1316938642) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly9hdWRpb2Jvb20uY29tL2NoYW5uZWxzLzQ5MzUxNjYucnNz) [PlayerFM](https://player.fm/series/the-doctors-kitchen-podcast)   The Gutology Podcast Bio The Gutology project is on a mission to help you optimise your gut. Join our gut health expert Julia Davies and award winning podcaster Ollie Gallant for a fascinating journey into your digestive system. We bring the science behind gut health and deliver it in 'digestible' chunks. This will change the way you think about your health forever. Julia Davies is a leading Nutritionist, SCENAR practitioner and lecturer in biomedicine and biochemistry. Ollie's digestive health started deteriorating in his early twenties and all of that changed when he met met Julia. They're now on a mission to help people do the same regardless of income, resource or location. [Apple Podcasts](https://podcasts.apple.com/gb/podcast/the-gutology-podcast/id1462264594) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly9ndXRvbG9neS5saWJzeW4uY29tL3Jzcw) [Spotify](https://open.spotify.com/show/5mMSRQRPJH4FECwrfIgZnz) Love & Guts Bio This platform is designed to speak to those who value living a full and magnificent life. Your host Lynda Griparic is a naturopath, nutritionist and yoga teacher with an avid and sometimes awkward interest in all things digestive health, especially bowel movements and the intelligent communication systems that exist in the body. Love and Guts explores all things mind-set, brain health, digestive health, pooping patterns, connection, communication and so much more. It's content may challenge you and at times it may get a bit whacky but never too bio-hacky as I like to keep things practical and accessible to all. [Apple Podcasts](https://podcasts.apple.com/us/podcast/love-guts/id1345246309) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cDovL2x5bmRhZ3JpcGFyaWMubGlic3luLmNvbS9yc3M) [PlayerFM](https://player.fm/series/love-guts)   Gut Health Gurus Podcast Bio A big welcome to The Gut Health Gurus podcast brought to you by Food Scientist, Kriben Govender and Psychology graduate, James Schadrach on a journey to investigate the gut brain connection. If you're fascinated by all things gut health, the microbiome, fermented foods, biohacking, optimal health & wellness and longevity then this podcast is for you. We bring you, various experts from around the world and explore topics from a scientific perspective. [Apple Podcasts](https://podcasts.apple.com/us/podcast/gut-health-gurus-podcast/id1433882512) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cDovL2d1dGhlYWx0aGd1cnVzLmxpYnN5bi5jb20vcnNz) [PlayerFM](https://player.fm/series/2431066) The Human Microbiome: Guts And Glory Bio A special series podcasts from NPR, concering the human microbiome, where trillions of bacteria, virus, fungi and other microbes hiding out in the human body shape health. [Podcast Link](https://www.npr.org/series/218987212/microbiome)   Feel Better, Live More with Dr Rangan Chatterjee Bio In this podcast, we hear stories from leading health experts and exciting personalities who offer easy health life-hacks, expert advice and debunk common health myths giving you the tools to revolutionise how you eat, sleep, move and relax. Hosted by Dr Chatterjee – a GP with over 16 years experience, star of BBC 1's Doctor in the House and author of The 4 Pillar Plan – Feel Better, Live More aims to inspire, empower and transform the way we feel. When we are healthier we are happier because when we feel better we live more. [Apple Podcasts](https://podcasts.apple.com/podcast/feel-better-live-more-with-dr-rangan-chatterjee/id1333552422?mt=2) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cHM6Ly9yc3MuYWNhc3QuY29tL2ZlZWxiZXR0ZXJsaXZlbW9yZQ) [PlayerFM](https://player.fm/series/feel-better-live-more-with-dr-rangan-chatterjee) The Gut Loving Podcast: All about IBS and the low FODMAP diet Bio The Gut Loving Podcast is all about irritable bowel syndrome (IBS) and the low FODMAP diet! The low FODMAP diet is a relatively new approach to effectively treat IBS symptoms and is scientifically backed up by a vast number of medical studies. The Gut Loving Podcast is hosted by Laura Tilt (an experienced Dietitian in the UK specialising in IBS and the low FODMAP diet) and Huelya Akyuez, patient and creator of project sezamee - gut loving food (video recipes and low FODMAP events) after living with IBS for over 20 years. Together, Laura and Huelya started The Gut Loving Podcast to help others with IBS get clear on the facts - and learn more about how to take control of their condition. [Apple Podcasts](https://podcasts.apple.com/us/podcast/the-gut-loving-podcast-all-about-ibs-and-the-low-fodmap-diet/id1278767162) [Google Podcasts](https://podcasts.google.com/?feed=aHR0cDovL3RoZWd1dGxvdmluZ3BvZGNhc3QubGlic3luLmNvbS9yc3M) [PlayerFM](https://player.fm/series/the-gut-loving-podcast-all-about-ibs-and-the-low-fodmap-diet)  --- This blog is powered by Superblog. Visit https://superblog.ai to know more. --- ## Ask the Doctor - Gut Author: BugSpeaks Published: 2019-03-29 Category: Others Meta Title: Ask the Doctor - Gut Meta Description:
In our previous blog on “commen
URL: https://www.bugspeaks.com/blog/commensals-and-cancer-therapy
In our previous blog on “[commensals and cancers](https://www.bugspeaks.com/blog-details?id=25&&title=Commensals and Cancers)” we tried to compile the updated information on relationship between gut microbiome and its effect on cancer (in general) and colorectal cancer (CRC) (in specific). Dysbiosis of gut microbiome is one the best studied associations for colorectal cancer, apart from its genetic and lifestyle related triggers. The scientific community has already deemed that the next decade of research in the field of oncology will be dominated by greater integration of microbiome and its impact, which will drive the invention and discovery of novel microbiome derived therapeutics of cancer.
Given this context, cancer therapy has also gained momentum in the recent times, with _reports of microbial communities within the gut modifying (both potentiating and weakening) the cancer drugs._ This has started a dialogue on the need for in-depth knowledge of the impact of the microbiome on cancer therapeutic strategies.
**Microbiome and Modulation of Cancer Therapy**
Human microbiome, specifically the gut microbiome has been extensively reported to modulate antitumor efficacy of chemotherapies and immunotherapeutic agents, in many pre-clinical models. It achieves it so largely in two ways; _“Modulate the immune system and Modify the drug or aid the process of such modifications”._
Through such modification the microbiome is said to either potentiate (increase) or weaken (decrease) the efficacy of immunotherapy and hence deemed essential for optimal drug efficacy of cancer drugs.

**Modification of CRC Drug (5-FU)**
Standard systemic treatment for CRC (metastatic and/or irresectable stage) is mostly based on fluoropyrimidine, such as 5-fluorouracil (5-FU). It is orally administered as capecitabine, or TAS-102. Capecitabine is a precursor of 5-FU, which must get converted to its active form to be effective against CRC. Many _in vitro_ studies have indicated a significant role of gut microbiota in this conversion. An increased response of CRC cell lines to 5-FU in presence of Lactobacillus _plantarum_ supernatant has been reported. 5-fluorocytosine (5-FC) samples, incubated with viable Escherichia _coli_ showed a higher increase in active 5-FU concentration. 5-FC, incubated with human faecal samples, also displayed a significant degradation reaction, indicating a significant increase in its potentiation. Hence associated with efficacy of drug and response.

On the other end of the spectrum, Colorectal cancers enriched in Fusobacterium _nucleatum_ have demonstrated worse prognosis. Because of the bacteria conveyed resistance to oxaliplatin and 5-fluorouracil by inducing [autophagy](https://www.nature.com/articles/nrd.2017.22). Oral administration of 5-Fu has been reported to alter the bacterial diversity and community composition of the gut. Several such instances have been reported involving both chemotherapeutic efficacy and chemotherapeutic cytotoxicity.

**Microbiome and Modulation of the Immune System**
Effector T lymphocytes represent a critical branch of the adaptive immune response to antigens. But, controlling the length and strength of this activation is very critical, which if unchecked can go damage even self-cells. Hence, a series of coinhibitory molecules, called immune checkpoints, are expressed by antigen presenting cells for switching off T cell activation.
Tumour cells inactivate cytotoxic CD8+ T cells, by expressing the same immune checkpoint molecules, and evade the anti-tumour immune response. Hence, targeting these immune checkpoints has emerged as a promising approach for cancer therapy. The most prominent one has been the class of drugs that act against programmed cell death protein 1 (PD-1) and PD-1 ligand 1 (PD L1).
However, patient responses to immune checkpoint therapies have been heterogeneous, varying with every individual and been reported to be transient. A cohort of 61 patients evaluated that PD-L1 expression in colorectal cancer defines three subsets of tumour immune microenvironments. Of which, third group, with low TANs, high IICs, both IICs and NCs PD-L1 expressing, would be the ideal candidate for this type of therapy. However, heterogeneous and transient responses to anti-PD1 therapy was still a concern.
In this context, studies have reported that the gut microbiota, possessing a pronounced modulatory effect on the immune system, may enhance responses to immune checkpoint therapies. Several Bifidobacteria species seem to stimulate dendritic cells (a kind of antigen presenting cells) in tumours, boosting the number of CTLs. This together have been reported to boost the response of anti-PD-1 and PDL1 drugs. This not only cleared the air about the previously observed heterogeneous and transient responses, but also established the impact of gut microbiome on cancer therapy.

**Faecal Microbiota Transplantation (FMT) and CRC Immunotherapy**
By definition, "FMT involves administration of faecal material (containing intestinal microbiota) from a healthy individual (donor) to treat dysbiosis and restore beneficial intestinal flora and phylogenic diversity.” FMT has proven to be beneficial in the treatment of recurrent Clostridium _difficile_ infection (CDI) and few other gastroenteric disorders.
Given that gut microbiome has such a significant on cancer therapy, it has recently found traction in cancer chemotherapeutic assistance. The transfer of highly complex whole communities of microorganisms from responders has been shown to result in durable changes in the recipient. FMT from colorectal patients into germ-free mice can elicit dysplasia and polyp formation. FMT from cancer patients responding to PD-1 based immunotherapies into germ free mice have shown increased efficacy. Non-responders to immune therapies were characterized by low abundance of Akkermansia _muciniphila_. Response to tumour therapy was improved by oral supplementation of the same bacterium.

**Conclusion**
The learning from these emerging studies, linking tumour development, progression, therapeutics and microbiome composition, is the fact that it is the microbiome which is key for developing personalized therapeutic approaches, since the microbiome composition of every individual is unique. At the same time, the shear complexity of the microbiome also adds several layers of complications to the precision medicine paradigm, but will no doubt drive the development of future therapeutics in oncology and other medical disciplines. The vision of utilizing a panel of bacterial marker species, that would designate an individual to be a responder or non-responder, is not too far from implementation, and will be checked before by clinicians to decide on personalized therapeutic approach. Overall, it is evident that the composition and diversity of the intestinal microbiota has significant implications in cancer treatments but will have to be thoroughly evaluated before implementation.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Gut Microbiome and Healthy Aging
Author: BugSpeaks
Published: 2019-03-08
Category: Microbiome and Lifestyle
Meta Title: Gut Microbiome and Healthy Aging
Meta Description: Ageing is an inescapable process. The health of an individual will inevitably deteriorate due to ageing and other physiological pr
URL: https://www.bugspeaks.com/blog/gut-microbiome-and-healthy-aging
Ageing is an inescapable process. The health of an individual will inevitably deteriorate due to ageing and other physiological processes. We cannot stop ageing, however in all probability, maintaining a healthy life and ensuring a “healthy ageing” is definitely in one’s hands.
World Health Organisation (WHO) defines healthy ageing “[_as the process of developing and maintaining the functional ability that enables wellbeing in older age_](https://www.who.int/ageing/healthy-ageing/en/)”. Functional ability is about having an optimal intrinsic capacity (mental and physical) and suitable environment (home, community and society). It is this intrinsic capacity that gets deteriorated over time with various internal and external factors, with immunological reasons being the most dominant one. Adding on to the abundant data that is already present, new reports have established strong connection between gut microbiome and human ageing and wellbeing.
**Microbiota and Immunosenescence**
As mentioned above, immunity and immunological reasons are the most dominant ones that hinder the process of healthy ageing. “Immunosenescence refers to the gradual deterioration of the immune system brought on by natural ageing process”. The ageing process is generally accompanied by a chronic (slow developing) background of inflammation, which is termed as “inflammageing”. A noteworthy negative outcome of [immunosenescence and inflammageing is characterized by a significant decrease in the functionality of immune system](https://www.ncbi.nlm.nih.gov/pubmed/28035338). This results in development of most age-related disorders, deteriorating intrinsic capability of individuals and significantly increasing the morbidity and mortality risk in the elderly.
_The human gut microbiota has a two-way interaction with processes of Immunosenescence._
On one hand, the microbial community in the gut (or the microbial burden) causing chronic, age-related inflammation is known to influence immunosenescence. While it is not conclusively proven that our gut microbiome is the root cause of inflammageing, there is a growing body of evidence to support this notion. The basic idea is that the gut microbiome is essential for normal immune function and development, and the immune system gets impaired by perturbations in the gut microbial ecosystem.
On the other hand, the process of immunosenescence has a significant impact on composition of gut microbiome, once it has reached a certain stage. This can cause troublesome changes in the composition of the gut microbiota, specifically in aged individuals. This usually results in the loss of bacterial diversity and a marked decline of beneficial microbial species during ageing, in turn leading to a compromised gut barrier, dysbiosis, increased bacterial infiltration into the body and eventually a vicious cycle of chronic inflammation.
Several studies on maturing gut microbiota have uncovered that the diversity of microbiota in elder individuals is significantly lower as compared to young individuals. It has been observed that a varied abundance of phylum Firmicutes and an overall low diversity in elderly subjects, is associated with increased health risks. [A distinct study](https://www.ncbi.nlm.nih.gov/pubmed/28035338) has reported significantly higher abundance of Bacteroidetes, and lower abundance of Clostridium cluster IV in elderly compared to younger subjects.
**Healthy Ageing through Gut Microbiome**
Since, the worldwide population is progressing towards a less dynamic, sedentary culture, it has become critical to prevent or delay age-related complications and maintain a good health. Many studies have explained that human wellbeing needs a valuable gut microbiota for physical and mental improvement at every age and alteration in physiological functions during the ageing process can affect the composition and functions of gut microbiota.
Hence, investigations have proposed that keeping up a decent gut microbiota through any means is critical for healthy ageing. But, the microbial community in the gut varies between individuals to a great extent and is dependent on eating habits, topography, host genetics, early microbial introduction and many other factors. Of all these, diet and nutrition are the most accessible factors that can be moderated by us, hence giving us control over our ageing.
**Nutrition, Microbiota and Ageing**
[Nutrition and diet play a significant role in shaping up of the host's gut microbiota](https://www.ncbi.nlm.nih.gov/pubmed/30025401). Nutrition is a key environmental factor that interacts with host genes, particularly in nutrient signalling pathways, and as recently reported, via gut microbiome. Hence, nutrition is a critical factor that can interlink gut microbiome with host genome.
Consuming high fibre or Microbiota Available Carbohydrates (MACs) containing diets or taking optimal mix and quantity of prebiotics species may help in [aversion and treatment of age-related pathophysiological conditions, which can aid healthy ageing](https://www.ncbi.nlm.nih.gov/pubmed/28035338) and advance life span. A high-fibre rich diet stimulates the gut microbiota towards performing different functions, as protection from inflammation, obesity, diabetes, heart disease, and high blood pressure. It has been reported that eating high-fibre content enhance the abundance of Bacteroidetes and decrease the abundance of Firmicutes. Studies also postulated that fibre-containing diets can help in developing the gut microbiota towards protecting the host from inflammation and non-infectious colonic diseases, due to the high abundance of _Faecalibacterium prausnitzii_ along with other Short Chain Fatty Acids (SCFAs) producing bacteria.
Various studies have suggested that butyrate, one of the products of SCFAs-producing bacteria, play several beneficial roles to improve the health in elderly. It has been hypothesized that the level of SCFAs, especially acetate, butyrate, and propionate, in the [gut of aged people is reduced compared to young subjects](https://www.ncbi.nlm.nih.gov/pubmed/27808595), and hence a diet that can aid production of SCFAs is critical. Nutrition and microbiome, together, also influences the host's epigenome; for instance, folate and choline, as dietary methyl-givers, can influence DNA methylation. A detailed account of various fibre rich foods and much more, that can aid in the process of building a healthy gut microbiome has been detailed [here](https://www.bugspeaks.com/blog-details?id=13&&title=Vegetables_and_Microbiome) and [here](https://www.bugspeaks.com/blog-details?id=14&&title=Fermented_Foods_&_Microbiome).

**Probiotics and Prebiotics in Ageing**
Consumption of [prebiotics](https://www.bugspeaks.com/blog-details?id=12&&title=On_Prebiotics) and [probiotics](https://www.bugspeaks.com/blog-details?id=15&&title=Kefir_-_Bridging_traditional_dietary_and_current_nutritional_practices) can augment the benefits of nutritious diet by multiple fold. Plant based diets, can elevate the abundance of beneficial species which is already present in your gut, or taken as probiotic supplement along with food. Prebiotics, which are also dietary substances that selectively promote proliferation and activity of beneficial bacteria indigenous to our gut, will also have similar effect on improving the composition and abundance of beneficial microbes. Other than these, numerous biomolecules, like micro-nutrients, fermented products, gut-related hormones and other compounds, are produced by these endogenous commensal organisms that are important in neurological and mental health of adults. [Together, they can promote growth, stimulate the immune system,](http://europepmc.org/abstract/med/28035338)[prevent gut-related and other diseases, which together is of critical importance for elder individuals](http://europepmc.org/abstract/med/28035338).
**Conclusion**
Overall, the strength of our immune system is supported by a healthy gut, which includes a complex interplay of symbiotic relationships between the gut microbiome and our body. Hence, developing, maintaining and sustaining a solid gut microbiome is the key factor to maintain a sound and healthy ageing, and this process is directly influenced by food and diet, probiotics and prebiotics. With [BugSpeaks®](https://bugspeaks.com), our endeavour is to provide you with a personalized, actionable and easily adoptable dietary, probiotic and prebiotic recommendation, which can aid you a long way through your ageing process.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Commensals and Cancers
Author: BugSpeaks
Published: 2019-02-28
Category: Microbiome and Disease
Meta Title: Commensals and Cancers
Meta Description: The last decade has witnessed a major surge in interest in the field of microbiology, which in turn has instilled a huge perce
URL: https://www.bugspeaks.com/blog/commensals-and-cancers
_The last decade has witnessed a major surge in interest in the field of microbiology, which in turn has instilled a huge perceptional change into the field of oncology (study of cancers)._
The surge in interest in the field of microbiology is due to the gut "commensals", the symbiotic microorganisms living with us. They have gained traction in the past decade for both their beneficial and detrimental influence on us, and significant impact on our health and wellbeing. This in turn has influenced a change in perception about cancers, both in its understanding and how to treat them. Even though, years of adopting molecular biology techniques, next-generation sequencing (NGS) technologies, whole genome sequencing and then multi-omics approach have aided us to understand cancer better than ever, the human microbiome and its influence on cancers have introduced a new paradigm to this understanding.
The link between microbiome and 'trigger, development & progress of cancer', and more recently even its treatment, has been well established. These established correlations indicate that the microbiome and cancer affect each other in a _“bidirectional and cyclical manner”_. On one end the cancer development changes microbiome composition while such changes also contribute to cancer progression in return, and this process continues in a cyclical manner. Furthermore, it’s also been established that the effect of microbiomes on cancers can both be detrimental (increase the pathogenesis of cancer and more) or beneficial (being protective against the triggers of cancer and more).
**Microbiome and Cancer trigger and progression**
Factors like gene mutations, family's genetic history, lifestyle habits (like smoking and alcohol consumption) etc., have long been studies and addressed with respect to cancers. Recently, there has been a rise in evidences associating involvement of microbiome (more specifically gut microbiome) in cancer. Initial studies revealed significant differences in the relative abundance of specific microbes in cancer cases compared with control subjects, which triggered a tirade of conversations and debates. Later, well-known associations between gut microbiota and inflammation & metabolism also got converged with its influence on cancer, expanding our understanding of contribution of microbiome in human cancer.

_Carcinogenesis is a process where tightly regulated systems of our body, such as immune activity and cellular metabolism, go off the track._ Either some perturbations in the microbiome or presence/absence of some specific microbial species/component or both can lead to such changes in cellular control.
These perturbations have been demonstrated to destabilize DNA integrity, deregulate signal transduction, modulate cell proliferation and even promote pro-tumorigenic (cancer inducing) immune responses. Some bacterial toxins can trigger senescent cells (cells that have stopped dividing) into proliferating cells by secreting growth factors. Specific microbial changes and its components have also been demonstrated to block tumour-killing capabilities of immune cells, enabling tumour progression and persistence even further.

Even though such activities have associated the role of microbiome with tumour development and have underlined several possible functional links, it is _still under debate if microbiome alterations can cause cancer or if they are merely associated with the disease_. But it has become critical to combine knowledge from _tumour-microbe-immune interactions_ with that of genomic, nutritional, pharmacological, social and behavioural effects of these interactions, for discovering and inventing next class of cancer treatments.
**Gut Microbiome and Colorectal Cancer**
Of all the cancers, human colorectal cancer (CRC) has been the one studied most, with maximum number of clinical studies and demonstrated associations between gut microbiota dysbiosis and the cancer. Specific bacterial species and strains have been associated with CRC, indicating inter-related links between gut microbiota, nutrition, immunity, tumour evolution and even tumour immunotherapy.

Fusobacterium _nucleatum_, Bacteroides _fragilis_ and Escherichia _coli_ were more abundant in worse prognosis groups, whereas the presence of Faecalibacterium _prausnitzii_ was positively associated with an improved survival. Of these, the most intriguing link between the gut microbiota and CRC has been associated with Fusobacterium _nucleatum_.
F. _nucleatum_ represents the best studied bacterium with a clear association with CRC. F. _nucleatum_ has been consistently found more abundant in faces from CRC patients, while its lower abundance has been associated with overall longer survival time. Even patients with simple adenomas exhibit an increased prevalence of F. _nucleatum_ in colonic tissue, while its persistence in metastatic colorectal tumour has been recorded.
Immunologically speaking, its abundance is inversely associated with CD3+ cell density in tumour, associated with serrated neoplasia pathway, increased expression both in high-grade dysplasia and established CRC. It may suppress the adaptive T cell response and may function in a way like the immune checkpoint in CRC. Quantitative PCR abundance of two gene markers (butyryl-CoA dehydrogenase from F. _nucleatum_) clearly separated the CRC microbiome form healthy controls. It is also been abundantly present with recurrence after chemotherapy and is known to promote resistance to chemotherapy.

Escherichia _Coli_ has the potential to cause intestinal inflammation via toxins such as colibactin, which also has oncogenic potential. This has been supported by various metagenomic studies in large CRC patient populations. Experiments in mice have demonstrated that host inflammation is essential for the cancer-promoting potential of E. _coli_. Mucosa-associated E. _coli_ is significantly more prevalent in CRC tissue, correlates with tumour stage and prognosis. Colibactin-expressing E. _coli_ is known to enhance tumour, accompanied by production of hepatocyte growth factor, overrepresented polyketide synthase pathogenicity island. Western diet affects enhances susceptibility towards the pathogenic potential of adherent invasive E. _coli_.
Bacteroides _fragilis_ is yet another human commensal found in the intestine of most humans. Bacteroides _fragilis_\-derived toxin (BFT) causes inflammatory diarrhoea and inflammation-related cancer. B. _fragilis_ toxin stimulates intestinal epithelial cell shedding and gammasecretase-dependent E-cadherin cleavage. Enterotoxigenic Bacteroides _fragilis_ (ETBF) induces colitis and colonic tumours in mice by secreting BFT. Dominated by inflammatory T cells and neutralization of IL-17 and IL-23 reduced inflammation and tumour formation. B. _fragilis_ colonization was paralleled by increased expression of TNF-gamma, ß-catenin, NF-kB, COX-2 and MMP9. Together with F. _nucleatum_ and E. _Coli_, B. _fragilis_ was more abundant in worse prognosis groups of CRC.
Klebsiella _pneumoniae_ may play a role in development of colorectal cancer. High prevalence in isolates from patients suffering from gastrointestinal diseases and CRC. Action through colibactin toxin and DNA strand breaks. Continuous insult of the colibactin toxin supplemented with the influx of pro-inflammatory cytokines. Colonic microenvironment leads to chronic inflammation. Repeated infection and insult of the colonic mucosa causes alteration in the structure and function. Stimulation of epithelial cell proliferation and thus colorectal cancer.

Other than these bacterial markers and associations, some metabolites can also be and have been used as diagnostic and prognostic markers, for its association with CRC.

**Microbiome and protection against cancer**
_On the opposite spectrum, the human microbiome has also been known to suppress tumour development and disease progression._
Like that of cancer trigger and progression, either some specific perturbations or presence/absence of specific microbial species/component suppress cancer and its progression. The mechanisms of suppression have been reported to range from augmentation dendritic cell function to increasing tumour-killing capabilities of cytotoxic T cells. But, more than these cellular mechanisms, it’s the metabolites generated by the microbiome which is known to play a major role in tumour suppression. For example, short-chain fatty acids (SCFAs) such as butyrate, generated by microbial fermentation of dietary fibres and resistant starches are extensively used as energy source by intestinal cells. However, tumour cells, which prefer glucose over butyrate, starve and suppress its growth or at least its progression at such low glucose conditions. Many such metabolites like butyrate (in gut) and others in skin has also been studied to reduce the incidence and multiplicity of tumours at various locations of the body.

Diet and nutritional habits have been strongly linked to CRC. This diet related risk is influenced by the balance between production of health-promoting microbial metabolites (ex. butyrate) and potentially carcinogenic metabolites (ex. secondary bile acids). Butyrate, a chemoprotective agent, acts as a histone deacetylase (HDAC) inhibitor capable of decreasing proliferation and increasing apoptosis in CRC cells. Butyrate could also diminish tumorigenesis by attenuating inflammation and colonocyte permeability and maintaining barrier function of the epithelium.
This relationship between the gut microbiota and CRC opens new approaches for cancer prevention. There are high quality experimental studies that yield the scientific evidences for the clinical use of probiotic in the prevention of CRC. Probiotic and prebiotic supplement can modify gut microbiota structure by reducing pathogenic gut microbiome and increasing probiotics.

A significantly decreased polyp number was also seen in mice given Lactobacillus rhamnosus GG (LGG). Consumption of LGG helped maintain the overall functional potential and taxonomic profile in the resident microbes. A 25% decrease of total polyp counts were observed. Further, LGG enriched those microbes or microbial activities related to short-chain fatty acid production (e.g. Roseburia and Coprococcus). Furthermore, suppressed the ones that can lead to inflammation (e.g. Bilophila _wadsworthia_).
Numerous studies have shown that iron significantly influences the intestinal microbiota, its composition and abundance. Of these, Bifidobacteriaceae are capable of binding large amounts of iron in the large intestine., thereby limiting the formation of free radicals synthesized in the presence of iron. This is known to reduce the risk of colorectal cancer significantly.

**To summarize**
It been irrevocably established that the human microbiome has a tremendous effect on the human illness and wellness. While the relationship between gut microbiome and its effect on various gastroenteric diseases were well known, its significant impact on cancer and its therapy has recently gained momentum. Dysbiosis of gut microbiome is one the best studied associations for the colorectal cancer, apart from its genetic and lifestyle related triggers.
Even though these associations are well established with various animal and human studies, our knowledge is still limited. This limitation comes from the fact that the impact of the microbiome on cancer is multifactorial, with a major uncertainty that if the dysbiosis of the microbiome is cause or an effect.

Even with these uncertainties, scientific community is optimistic that the next decade of research in the field of oncology will be dominated by an even greater integration of microbiome and its impact, which will drive the invention and discovery of novel microbiome derived therapeutics of cancer.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## A Letter from Liver
Author: BugSpeaks
Published: 2019-02-22
Category: Microbiome and Disease
Meta Title: A Letter from Liver
Meta Description: Hi, I am your happy happy liver. I am the filter of your body. I filter and detox your body f
URL: https://www.bugspeaks.com/blog/a-letter-from-liver
Hi, I am your happy happy liver.
I am the filter of your body. I filter and detox your body from harmful substances that come in and allow only those which are needed. So, I am pretty important to you.
Specifically, I am a major filter of nutrients absorbed by the intestines and I am also the filter of microbiota generated by-products, filtering in the beneficial ones and filtering out the deleterious ones. Me, I am exposed (read bombarded) with these bacterial components and metabolites on a regular basis. One of the perks (or perils) of being so close to the intestine.
_**I have a two-way or cyclical connection with the gut, and in context, specifically with its microbiota.**_
Me and the intestine communicate extensively through the biliary tract, portal vein and systemic mediators. So, as I said, this begins with intestine passing on the digested metabolites and toxins to me and I filter in or filter out. For instance, some components of gut microbiota called Pathogen Associated Molecular Patterns ([PAMPs](https://www.ncbi.nlm.nih.gov/pubmed/25492994)) and Microbial Associated Molecular Patterns (MAMPs) needs to constantly filtered in or out. Some of these are endotoxins (lipopolysaccharide or LPS) that are deleterious and hence filtered out, and some are bacterial metabolites (examples) that are beneficial, hence filtered in. On the other hand, many intestinal factors regulating the bile acid production, glucose and lipid metabolism in ‘yours truly’ (liver). And… the cycle continues with my (liver) products, such as, influencing the gut microbiota composition, intestinal integrity and much more.
Things get messy when the [gut microbiome dysbiosis](https://www.ncbi.nlm.nih.gov/pubmed/27802157) happens (as in variation in gut microbiome composition and abundance), along with multiple interactions with human’s immune system and other cell types. This dysbiosis can cause imbalance in our otherwise harmonious communications, which in turn can have some deleterious effects on me (Well… just like any other relationship).
_**So what does the relationship counsellor say..?**_
They say there is some research that have already reported and much research going on linking my disorders and conditions to gut microbiota. It is now widely accepted that the damage caused to me (liver) can result from [extensive interplay](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6319369/) between the gut microbiota produced specialized molecules (such as TMA, acetaldehyde and LPS) and the host immune system, via Kupffer-cell-mediated liver inflammation.
I know, I know
This is too technical
But, let me explain what I have understood, taking an example.
Incidence of Non-alcoholic fatty liver (NAFLD) is globally rising and it is a common multi factorial liver disease. This is the condition where, I become inflamed and huge, scarred and scaled, and I don’t like myself like this.
_**How is this caused?**_
Remember the endotoxins? the deleterious things I filter out?
Some initial disruption of the intestinal integrity increases the permeability of its walls that leads to increased bacterial translocation, which in turn increased that load of bacterial endotoxins that penetrate the portal vein (more than I can handle). These endotoxins are recognized by toll-like receptors (TLRs) on hepatocytes (my cells). When bacterial LPS signals through TLR4, signalling ultimately activates NF-κB and proinflammatory cytokines, and initiate a barrage of immunologic responses. Together, this increases the risk of [NAFLD](https://www.ncbi.nlm.nih.gov/pubmed/26449892) development through the activation of hepatic inflammatory cells (that’s me!).

_**So, intestine is the cause**_
_**Why are u still in this relationship then?**_
Well… as it almost always happens in a relationship, when one door closes, another opens. What seems to be cause for my demise, the gut microbiota, is in itself the cure.
Firstly, increasing reports of links between NAFLD and gut microbiome, both at observational and at mechanistic levels, have indicated them as an attractive source of biomarkers for early diagnosis of NAFLD.
So, the microbiota that cause my disease, are also used to identify if I will get the disease.
That’s a good thing.
In fact, serval gut microbiome-based tests are already available, which can understand what your [BugSpeaks®](https://bugspeaks.com/) about your health and provide you with risk estimations of diseases that you may be predisposed to. This can be used later to confirm the findings with physicians and clinicians, and even get personalized recommendations.
_**Now to the cure or alleviation or protection of my disease**_
A major beneficial role of the gut microbiota in liver disorders is also supported by [accumulating evidences](https://www.ncbi.nlm.nih.gov/pubmed/30227645) that several complications of severe liver disease, such as hepatic encephalopathy, are efficiently treated by various prebiotics and probiotics. Gut [microbiota manipulation](https://www.ncbi.nlm.nih.gov/pubmed/28298269) through nutritional factors, with [prebiotic potential](https://www.bugspeaks.com/blog-details?id=12&&title=On_Prebiotics), with additive [probiotics](https://www.bugspeaks.com/blog-details?id=15&&title=Kefir_-_Bridging_traditional_dietary_and_current_nutritional_practices), and supplementary polyphenols can alleviate the characteristics of NAFLD.
Of these, dietary factors are strong predictors of the gut microbiota composition. In fact, it has been projected that dietary factors play a more important role in shaping the gut microbiota composition than do genetic factors. Nutritional factors and the gut microbiota have bilateral interactions that contribute to the development and/or protection from NAFLD.
Most importantly, gut microbiota using prebiotic and fermentable carbohydrates as energy sources, have major beneficial impact on NFALD. Short Chain Fatty Acids (SCFAs), such as propionate, produced through this fermentation, cross the gut barrier and reach the liver through the portal vein blood. Propionate inhibits lipogenesis by acting on the transcription of several rate-limiting enzymes involved in _de novo_ lipogenesis. Consequently, these fibres and their metabolites are putative tools to reduce steatosis and inflammation.
_**Hence, I am saved. Phew!**__**!**_
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## How changing a body's microbes leads to illness
Author: BugSpeaks
Published: 2019-02-11
Category: Microbiome and Disease
Meta Title: How changing a body's microbes leads to illness
Meta Description: Claire M. Fraser, PhD, in this TED talk explains how microbes affect our lives. We are more obsessed with cleanliness than ever: we use antibacterial cleanse
URL: https://www.bugspeaks.com/blog/how-changing-a-body's-microbes-leads-to-illness
Claire M. Fraser, PhD, in this TED talk explains how microbes affect our lives. We are more obsessed with cleanliness than ever: we use antibacterial cleansers, we keep our children away from dirt, and we give out antibiotics with little regard for long-term effects. But in the process, we are altering our microbiota, the microbes that live on and inside us. And it's causing us to be more sick.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Diet, Digestion and Microbiome
Author: BugSpeaks
Published: 2019-02-11
Category: Diet and Supplements
Meta Title: Diet, Digestion and Microbiome
Meta Description: Professor Kevin Whelan talks about diet and the microbiome at Core's Exploring the Science of Digestion event at the Kia Oval, London. The human body comprises 10-100 trillions of microbial cells, including archaea, bacteria, viruses, eukaryotes. The micr
URL: https://www.bugspeaks.com/blog/the-human-gut-microbiome-and-microbiomics:-progress-towards-personalized-healthcare
The human body comprises 10-100 trillions of microbial cells, including archaea, bacteria, viruses, eukaryotes. The microbiome includes the combined genetic material of all microbes in a specific environment i.e., microbiota. It plays a significant role in improving human health and preventing diseases in many ways, though their mechanisms are yet to be probed in detail. The occurrence and function of microbiota differ depending on several factors, such as ages, sexes, races, diets, and change in the locations of the host. The microbiome profoundly restores normal phenotypes in the host. The past few years have investigated the microbiome in detail, and the results have tremendously reshaped our knowledge of human biology. Some of the innovative insights range from the understanding of how microbial cells intervene digestion activities, disease initiation, and progressions (e.g., inflammatory bowel disease) to unforeseen links with autism, depression, anxiety/mood disorders, Alzheimer's, Parkinson's disease, etc.
So far, studies have discovered around 10,000 microbial species (mainly bacteria) in the human body and mostly, their occurrence is predominant in the gut. Interestingly, the gut microbiota contains about 150 times more number of genes than that are observed in the whole human genome. Thus, the gut microbiome is sometimes recognized as an ‘essential organ’ because it produces products, it responds to the environment, and interacts with other systems. It is believed that the impact of these bacterial genomic DNA on our health might be more than our own genomic DNA. Likewise, it has been realized that the microbiota of gut and brain are intricately associated. There is an exchange of messengers/signals between the brain and gut microbiota. The entire body cells are influenced by the microbiome in one or the other way. In specific, it affects our mood, metabolism, libido, immune system, sensitivity, and even the clarity of your thoughts. Scientific evidences have shown that human health and diseases are dictated by what goes on in the microbiome of the gut. Diseases such as inflammatory bowel disease (IBD), obesity, diabetes, cardiovascular diseases, atherosclerosis, nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), cirrhosis, and colorectal and liver cancer have been connected to the activities of the human microbiota (Figure 1).

**Figure 1. Human microbial symbiosis has a close relationship with diseases of different systems (Adopted from Wang et al. 2017; [https://doi.org/10.1016/J.ENG.2017.01.008](https://doi.org/10.1016/J.ENG.2017.01.008)).**
Overall, the diversity in microbiome can lead to both health and illness. Commensal microbes colonize the host soon after their birth. This microbiota community gradually develops with the host growth depending on varying ecosystems. Over time, these host-microbe interactions lead to a beneficial association, and symbiotically offer many advantages. For example, improvements in digestion, supply of essential nutrients, metabolizing indigestible compounds, and to shield against the invading pathogens. Knowing about the microbiota can help in preventing possible diseases in humans. However, their complex nature makes it difficult to study them. The standard microbial culture methods may not be sufficient enough to isolate and explore all microbes present, because some may not grow or require special growth conditions that are yet to be standardized. Later, culture independent methods for evaluating microbes was developed based on the targeted sequencing of 16S and 5S ribosomal RNA (rRNA) genes which showed differences for each species and effective in identifying organisms. Over the last decade, human microbiome researches have been transformed by using next-generation sequencing (NGS) technologies. These high-throughput sequencing allow us to study complex bacterial systems without the requirement of cloning individual genes. The targeted sequencing of 16S rRNA, 18S rRNA, 28S rRNA genes, and internal transcribed spacer (ITS) regions, and whole microbial genome of microbial communities all at once (metagenomics) is very useful in the identification of microbes and to track the functional activity of the entire community. The increasing number of such metagenomic studies of microbiomes in humans is a new hope of its potential benefits in the clinical practice.
Recent studies have witnessed the occurrence of human genetic variations as influenced by interpersonal dissimilarities in microbiomes. Thus, human genes might influence health by promoting beneficial microbiomes. The heritability study of the gut microbiota has revealed that subsets of microorganism’s abundance are genetically determined by the host. For example, a genetic change enables lactase persistence in adulthood. Interestingly, the genome-wide association studies (GWASs) of the microbiome has revealed that there is a relationship between the host genotype, consumption of milk, and gut beneficial bacteria (Bifidobacteria). It has been noticed that a single nucleotide polymorphism (SNPs) near the LCT gene on chromosome 2 and Bifidobacterium in the fecal microbiota has a close association. Bifidobacteria utilizes the lactose of milk as an energy source. This depends on the genotype of the host and diet interaction (Figure 2).

**Figure 2: Interaction between the human lactase nonpersister genotype, Bifidobacterium abundance, and lactose in the host’s diet. Genetic variants near the LCT gene are associated with lactase persistence and in several microbiome GWASs were recently associated with Bifidobacterium relative abundance in fecal samples. The association may function according to the following scenario: (a) If an individual is a lactase persister and consumes lactose, the lactose is typically broken down into glucose and galactose in the small intestine by host lactase. (b) However, if the individual is a lactase nonpersister and consumes lactose, it travels to the large intestine, where it is fermented by lactose-utilizing bacteria, which includes Bifidobacterium. If Bifidobacteria are present, then the presence of lactose promotes their abundance. In individuals who do not consume lactose, Bifidobacterium abundance remains unaffected by their lactase persistence status (not shown). (Adopted from Goodrich et al. 2016; [https://doi.org/10.1016/j.cell.2014.06.037](https://doi.org/10.1016/j.cell.2014.06.037)).**
Likewise, GWASs has revealed that immunity related genes are influenced by the gut microbiome. Evidences suggest that genetic variations might act on digestive tract tissues to disturb the composition of the gut microbiome. Further, genetic variants in the genes, PLD1 and LINGO2 are associated with obesity. Based on the analysis of 16S transcripts, the phylogeny of microbial community has been revealed. The most active microbiota included Prevotellaceae, Lachnospiraceae, Bacteroidaceae, Ruminococcaceae, and Rickenellaceae. RNAseq analysis of bacteriophage populations in the periodontal microbiota showed the abundance of oral phages highly expressed in persons having good periodontal health. More recently, 16S rRNA gene-based approach has confirmed the predominant occurrence of Corynebacterium spp., Staphylococcus spp. and Propionibacterium spp. on healthy human skin.
Thus, knowledge on the microbiomics help to improve human health conditions. A defined diet can influence on the microbiome and restore health. Likewise, a proper use of chemicals/drugs in disease management is another factor to be considered. For example, both foods and drugs are used in treating type 2 diabetes. It is generally advised to avoid consuming simple carbohydrates to control blood sugar. Nevertheless, there exist inter-individual glycemic variations in response to diet, and some of which can be described on the basis of the individual’s microbiome. Similarly, the human microbiome plays a significant role in genetic diversity, modify disease conditions, impart immunity, provide nutrients, influences assimilation, and modulates drug interactions. Thus, intake of probiotics or beneficial bacteria (Lactobacillus, Bifidobacterium, Faecalibacterium prausnitzii, Akkermansia muciniphila, Pediococcus pentosaceus, etc.) may be useful in preventing or treating certain diseases, though most of them cannot be cultured presently. As a diagnostic tool, microbiomics is employed for detecting and measuring the levels of biomarkers i.e., cholesterol, cortisol, glucose, and creatine kinease occurring in blood which are known to indicate about diseases or infections. These novel diagnostic methods will allow clinicians to prescribe right drugs or diet to restore patient’s health.
Future microbiome investigations should involve many more new techniques to predict the functions of microbiotas, their crucial role in human development, and the mechanisms of various diseases, such as metabolic diseases, liver diseases, cancers, psychiatric diseases, etc. In this regard, many new-startups and biopharmaceutical companies, including Luecine Rich Bio Pvt. Ltd are exploring the field of microbiomics to develop novel therapeutics, diagnostic platforms and to provide clinical genomic services. A detailed data on this metagenomics might be very useful in the microbiome-based diagnosis of diseases and for the development of novel treatment strategies in the forthcoming personalized medicine era.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## BugSpeaks through Cladogram
Author: BugSpeaks
Published: 2019-02-04
Category: On Diversity Debate
Meta Title: BugSpeaks through Cladogram
Meta Description: Charles Darwin, with this famous entry in his journal, begins with "I think" on the top left-hand corner of his notebook
URL: https://www.bugspeaks.com/blog/bugspeaks-through-cladogram
Charles Darwin, with this famous entry in his journal, begins with "I think" on the top left-hand corner of his notebook and then sketched out the first “tree of life”, which was his perception of how all living things on earth are connected through evolutionary history. Through this he proposed the theory of "the origin of species through natural selection" and divergence of all species from an ancestral population.

In short, he meant we all are related!!
That doesn't mean only us humans. But every living being on earth can trace its descendance back to a common ancestor.
To understand this, let's begin with basic taxonomic classification, which we all studied during our biology 101 classes. By definition, _“Taxonomy is the science of defining and naming groups of biological organisms based on shared characteristics.”_ Organisms are grouped together into groups termed as ‘taxa’, and these groups are given a taxonomic rank, which looks something like this
**Kingdom => Phylum => Class => Order => Family => Genus => Species**
Now, when you want to show these relationships among organisms in a simplified way, the phylogenetic tree comes into picture, through which relationships are illustrated by a phylogenetic tree/dendrogram.
**Phylogenetic trees**
represent hypotheses about the evolutionary relationships (the descendance) among a group of organisms, originating from a common ancestor and can be built using morphological (body shape), biochemical, behavioural, or molecular characteristics of species or other groups. Dendrograms are often used to illustrate such evolutionary relationships, along with information like _evolutionary time line_ separating the taxa (like, how many million year ago did this species come into being), _genetic distance_ (between the gene sequences being classified), and so on.
**A cladogram** (from Greek, clados "branch" and gramma "drawing") is a type of phylogenetic tree that only shows tree topology. Basically, a cladogram uses lines that branch off in different directions, representing a group of organisms with a last common ancestor, ending at a clade. The branching pattern is called the tree’s topology.

There are many shapes and forms of cladograms, through which the phylogenies are represented, but they all have lines that branch off from other, and the lines can be traced back to where they branched off.
By topology, it also means showing only the basic relationships between the taxa, without any additional details. For example, say we want to represent human evolution through a cladogram, then we represent chimpanzees to be more closely related to us than gorillas. But this is represented just as a tree, without the time line (unlike the pictorial representation besides).

Now let's take a closer look at the cladogram, where we will zoom-in on one clade within the cladogram. Always, the species (taxa) are found at the tips (represented by B, F and C), extended by lines referred to as the tree's “**branches”.** The pattern in which the branches connect represents our understanding of how the species in the tree evolved from a series of common ancestors. Each node represents a **divergence** event or splitting of one group into two descendant groups.

Take a look at this amazing [candy bars](https://wildlifesnpits.wordpress.com/2014/03/22/understanding-phylogenies-terminology/) phylogeny that shows the relatedness between candy bars represented as a cladogram. This draws an accurate analogy to understand the basic evolutionary relatedness of species (and has the added advantage of candy bars being easily accessible (unlike animals) and easy to understand the phylogenetic tree structure even by a layman).

These candy bars are related to each other based on their content and shape. In a similar way species can also be compared and represented based on their genetic content (DNA/RNA) and their morphological characteristics. If we dig deep, the peanut clade has three candy bars or taxas (representative of species) with peanut as its common ingredient (representative of common ancestor). With such analogies we can understand how phylogenies are connected and important to understand the evolutionary insights.
**Cladograms and Microbiota**
Mainly phylogenies are used to represent species evolution, as in of higher organisms like humans, fishes and tress and so on. But there are also other applications like, plotting microbial taxonomic compositions of metagenomic data, which is the base of our [BugSpeaks](https://bugspeaks.com/)® analysis. In the specific context of microbial genomics and metagenomics, next-generation sequencing data, of say gut microbiome, produces datasets of unprecedented size and tremendous genetic diversity of microorganisms. And cladogram seems to be the simplest way of displaying these diverse phylogenies with thousands of microbial taxa within the sample.
Since cladograms are simple topology-based representations, they are simpler to understand by the end users and does not complicate the data with too much scientific information. However, we have also made specific modifications to these representations, to not just represent microbial composition, but also to display it’s metadata like species abundances, host or environmental phenotypes etc. With the end user in mind, just this simple cladogram can present the overall microbial diversity and other relevant information, yet also provide specific scientific data (like alpha diversity) of the sample, if the same representation is viewed by a researcher or a clinician with microbiome background.
Let’s try to read one such annotated cladogram representing microbial composition of a human gut sample.

This is a circular cladogram with basic visualization of the tree’s hierarchical structure. It has all the same representations as nodes, branches and taxas that were discussed above. It has four major clades (kingdoms) starting from centre, including Archaea, Bacteria, Eukaryota and Viruses presented in four different colours. Each has branches which represents next levels (Linnaean ranks), including Phylum, Class, Order, Family, Genus and finally the tip representing species (this cladogram image shows family level phylogeny). The size of circles at nodes and tips represents the abundance of rank and outer ring represents the species abundance in the form of bar plot (grey bars on the outermost circle). Highly abundant taxas are annotated as legends at top left corner.
By providing such a cladogram, we give the overall picture of an individual’s microbial diversity in his/her gut. Supplementing this with other plots and graphs, we provide a thorough summary of one’s [BugSpeaks](https://bugspeaks.com/)® profile, all represented through pictures.
Coming a full circle, what Darwin sketched as the simplest idea of tree of life, is still relevant and applicable even after a century, which is only a testament to his genius.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Diabetes and Dysbiosis: A tale of two Diabetics
Author: BugSpeaks
Published: 2019-01-29
Category: Microbiome and Lifestyle
Meta Title: Diabetes and Dysbiosis: A tale of two Diabetics
Meta Description: Below are some ‘Keyword’ searches for ‘diabetes’ being googled daily by millions of people across the glob
URL: https://www.bugspeaks.com/blog/diabetes-and-dysbiosis:-a-tale-of-two-diabetics
Below are some ‘Keyword’ searches for ‘diabetes’ being googled daily by millions of people across the globe.

And it is for a reason…
Diabetes Mellitus Type 1 & 2 or largely known just as ‘Diabetes’, is a rampant chronic (slow developing) disease around the globe. It’s also one of the most searched because of increasing number of risk factors for diabetes and diabetes being the risk factor for many other diseases. It has significant effect on body and mind of individuals and greatly reduces their quality of life.
Coming back to searches, since we all believe Dr Google so much, and almost always end up searching (and believing) anything and everything about the disease we are diagnosed with. But we also know that most of us won’t succeed in even understanding the disease, let alone managing it well.
And there begins a tale of two diabetics….
**Meet Mr A**
An everyday common man, taking his car to work all the way across the city. The to and fro daily commute nearly leaves him drained. His 8 to 8 job makes him too tired to get up early and hit the gym. His desk job is convenient and is just an exercise of the mind. His weekends usually pass by doing house chores and shopping, a movie, may be a resort spa outing, some relaxation and back to Monday blues. His life is great and the biggest problem he currently faces is slow moving traffic and being conscious about his stomach paunch at 32.
As life goes by, he begins to feel tired all the time, fatigue becomes a daily thing, hungry in between meals, heightened craving for sweets, chips and snacks, too drowsy usually an hour before his lunch, forced to have early lunches and so on. This goes on for few months to about a year.
On a seemingly normal day, a routine check-up and blood test at the company (because he is too busy being busy for these check-ups to be a regular part of his life), it’s revealed that his blood sugar is off the charts, and he is diabetic.
This rings a bell in his head, and he thinks, now is the time to change and turn my life forever. And the first thing he does… search (not read) anything and everything about diabetes on the internet.
A series of statistics pops up, stating diabetes is so rampant, with close to 422 million people being affected worldwide. With the first few searches on how to fix it, he gets that his lifestyle needs some serious changes and that too immediately. No junk food, balanced diet and vigorous exercise; the whole package. He thinks, may be walking and working out at a gym everyday should become part of his routine.

Then he digs a bit further, and starts to read about glycaemic index, and ...
what if the disease was diagnosed late?
what if it’s progressed far?
what if, if I can’t get my sugar under control, even with all the new diet and exercise routine I am planning.
Oh my god, my insulin production will get depleted at an alarming rate
I might have to take insulin intravenously to supplement my glucose metabolism
my quality of life will dangerously be deteriorated, and my family & friends will be concerned too much
Oh, this search also says I might get other disorders, liver problems and heart diseases, my kidney function could get affected forever, with increased risk for glaucoma
Oh damn, here it says if I get a cut on my leg, the wound will never get healed, it will get infected, and the doctors will have to amputate my leg
Oh god… Oh god… what will I do now
And… this downward whirl of “what-ifs” goes on for a while, and he gets so worried… so worried… that…
….he goes to sleep.
Next day morning, he goes back to his “so called regular life”.
**Now, meet Mr B**
Mr B on the other hand, having a similar lifestyle was also diagnosed with diabetes a while ago. He too, like most others, searched the heck out of google for all possible causes of and cures for diabetes. But, being a sensible man he is, without self-instigating much of panic or pressure, he starts to gather relevant information from credible sources, like doctors, clinicians and nutritionists. He too gets to know about glycaemic index and glaucoma, lifestyle and dietary changes that he needs to adopt, and most of the other information that Mr A got, but with actionable recommendations that are largely easy to implement in his lifestyle.
He also understands that, the long-standing notion that diabetes is only caused due to genetic factors or is largely lifestyle related (unhealthy diet, overweight, physical inactivity etc.), has been recently questioned. With the new and rapidly developing field of microbiome research, several new links have been established between [gut microbiota](https://www.gutmicrobiotaforhealth.com/en/digging-into-the-hidden-world-of-the-microbiome/) and diabetes, not only to its causation but also to the drug efficacy, alternate forms of treatments and many more.
And suddenly an interesting phrase catches his attention
_Local communities, Global Impact_
He reads again,
_Local communities, Global Impact_
He realizes, for the first time, that there are trillions of tiny tenants living within his gut, forming several local communities, have a global impact on our body and mind, and have been known to play significant role in disease and health.
Now he is intrigued, and his interest is peaked…
And he continues to understand more.
The gut microbiota affects numerous biological functions throughout the body and its characterisation has become a major research area in biomedicine. Studying the relationship between microbial alterations within the gut and functional repercussions linked to disease(s) remains challenging, and even more complex when it comes to their interactions with therapies and drugs. Animal studies and other human trials have associated microbial patterns in individuals with diseases and traits, thereby implicating gastrointestinal microbial communities in a range of human disorders.
Oh… all this can happen from my gut? Inside out?
The complex relationship between [obesity](https://www.ncbi.nlm.nih.gov/pubmed/20876708), diabetes, and gut microbiota is being uncovered, with consistent growing body of evidence. Presence/absence, relative and/or absolute abundance of specific microbes within the gut has been shown to have a direct impact on insulin sensitivity and glucose metabolism, adding an important dimension to understanding of diabetes.

Now this is way too technical for me…
He quips!!
But he continues…
Then came a bunch of biological names like Collinsella, Oscillospira, Akkermansia (most of which he could not pronounce), followed by some more bio jargon about pathways and stuff. All he could get from the picture besides was the burger and high-fat diet. He also gets fascinated by realizing that [metformin](https://www.ncbi.nlm.nih.gov/pubmed/26633628), which is one of the most common medication given to manage diabetes (which was also prescribed to him), not only helps to reduce the amount of sugar the liver releases into the blood but could also aid by improving the gut microbiome.
The most relatable thing that he grasps, by these searches and readings, was the extensive information about probiotics. It seems like [probiotics](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4402713/) can help a whole host of gut and other issues. Even though it is not proven to have absolute beneficial effect on diabetics, efficacy of probiotics in diabetes has been proven by their ability to lower fasting glucose and insulin levels in various. The link between gut dysbiosis and diabetes has been convincing and promising, particularly since probiotics have been shown to normalize the disturbed metabolism in diabetes patients.
he feels a sense of optimism...
but with a disclaimer that,
It’s hard to rule out the effect of other factors that may have affected glycaemic control in these [studies](https://www.nature.com/articles/nm.4345), yet, this link between probiotics and alleviated diabetes is a promising new supplementary to metformin treatment, in lowering fasting blood sugar.
…and regarding probiotics, his take home message was that, probiotics undoubtedly have an overall positive outcome on diabetics, and its can greatly supplement the main treatment options to manage diabetes well for a long time.
Overall, he got the gist of it.
That,
His gut microbiome and gut health can greatly influence diabetes and by understanding and managing his gut well, he can manage his diabetes too.
Now, he took Google’s help (the right way this time around) to find a [microbiome-based company](https://bugspeaks.com/) providing testing services and evidence-based interpretation of gut microbiome, which can help him better understand and guide him to make optimal effective changes to his health and lifestyle. He sent his stool sample to the testing facility, followed up well, and received an extensive report, including his disease susceptibilities (diabetes and others), as set of customized dietary, probiotic and dietary recommendations, follow up with nutritionists and clinicians for further advice, overall a complete package.

Having diagnosed early and his sensibility to understand and rely on credible sources, and to explore new avenues of diagnosis and treatment, aided Mr B with effective management strategies for diabetes. He inculcated all the necessary exercise and diet much early into his diagnosis, he took the help of nutritionists and turned around his life for the better, supplementing it with exercise and improved lifestyle. Now, that’s what we call a happy ending!

---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Obesity is Contagious
Author: BugSpeaks
Published: 2019-01-25
Category: Microbiome and Lifestyle
Meta Title: Obesity is Contagious
Meta Description: Let’s begin with this 140 So, it’s been two months past the new year. We all are coming back to our normal selves, we are eating (binging) whatever we
URL: https://www.bugspeaks.com/blog/what-happens-in-vagus,-does-not-stay-in-vagus
So, it’s been two months past the new year. We all are coming back to our normal selves, we are eating (binging) whatever we want to, not so keen on working out or even waking up early...for that matter.
Now that we are enlightened about new year diets, workout regimes & resolutions, and have understood these to be just myths, we can actually focus on doing what we do best
"**sit and ponder about everything**"
Everything, from menial questions about career, marriage, relationships ... to ... more serious questions like - should I cook today or order pizza?... then to ... potentially life changing questions like... Why am I fat ?!!
We always talk about stuff that we eat going directly to our hips (especially for us girls, right?!)
Here is an interesting observation:
What if, the food we eat directly goes to (or affects) our head?
hmmm... Now that's a thing to ponder upon,
I mean how's that even possible?
Well, you might have heard or even felt that, when you are in stress you eat more, or when you are tensed you have gastric problems, or when you are hungry for long duration you get a headache. So, you know these things are somehow connected, and mostly seem to be related to the food that you eat.
But, in fact, this relationship, between your behavior and your food (more importantly the other way around), is surviving (read hanging in there) because of the tiny tenants in your body.
_**And this is where the story begins...**_
When you are in your mother's womb there are no microorganisms in your body, and you are virtually sterile, inside out!!
As a new-born, you are exposed, for the first time, to a wide array of microbes from a variety of sources, including maternal bacteria. The process of birth exposes you to the first microbes from your mother's vaginal tract or stomach, depending on the mode of birth. (fascinating? or you feeling eeeww?)
Later, your mother's milk gives you the major share of microbes, that actually help you build your immunity. In fact, [a recent study](https://www.ncbi.nlm.nih.gov/pubmed/23401408) talks about how infants born by cesarean section are at increased risk of asthma, obesity and Type 1 diabetes, as compared to babies born through normal labor and exposed to the maternal vaginal microbes.
As and when you start eating different kinds of foods, drinking beverages, use a specific kind of bathing soap etc., the microbiome (total microbes on and) in your body gets enriched. More specifically, your gut microbiota gets developed with every exposure to different environments. In fact, what you eat, drink, smoke, or gets exposed to, all defines the microbiota of your gut.

I still remember the day, when I had my first beer... and my gut microbes were like... bring it on girl!!
Another interesting fact is that, since diet and food habits are largely an extension of your culture, your gut microbiome differs from American's or Chinese, given that we all are culturally world apart, and so is our lifestyle and dietary habits.
_**The other side of the story…**_
On one end, the food you eat and drink affects the composition of your gut microbiota. However, over time, the enriched and developed gut microbiota influence the digestion of specific food you eat, indirectly influencing your diet. So, lot of these gut bacteria influence and more importantly help you digest food in an efficient way.
Another interesting fact is that, whenever these good bacteria goes low in number, it gives way for over growth of bad microbes like Clostridium, E. coli etc., that causes diseases like diarrhea, colitis, etc.
And why would these good bacteria go low in number?
Well... it can be due to any reason from imbalanced or untimely diet to exposure to antibiotics or smoking. [One study found](https://www.ncbi.nlm.nih.gov/pubmed/3846592) that after a single treatment of intravenous antibiotics, fecal bacteria demonstrated a significant change in the variety and abundance of bacterial strains.
Overall, the food that we eat influence the gut microbiota and the microbiota influence the what we eat - requiring a fine balance between the two.
Okay, now we are drifting
_**Let's come back to the point where food and head are connected**_
Studies, involving college kids (the junk eating generation), eating burgers and fries, guzzling cokes and sodas, observed that these students felt lethargic, largely unwell, depressed, with general fatigue, disturbed / unfocused mind, just after a few weeks of continuous ingestion of the junk diet. Testing their gut revealed that, and I quote “gut microbes (the microbiome) had been devastated”, with potential disease causing firmicutes replacing good Bacteroidetes, as the dominant type, while the friendly Bifidobacteria, that suppress various kinds of inflammation, halved in its abundance being the clearest marker of an unhealthy gut.
The question is why? and How does what you eat .... affecting your gut microbes... affect your state of mind?
The human gut microbiome is so dynamic and complex, that it consists of approximately 1 kg of bacteria in an average adult, and is approximately the weight of the human brain. This microbiota produce a myriad of neuroactive compounds, regulating everything from your behavior to cognitive function, social interaction to stress management. It regulates these brain functions by steering the "Vagus Nerve".
This thing called 'Vagus nerve' (Not "Vegas and Casinos") within your nervous system, is a part of cranial nerves unit, that forms an extensive network, linking your gut and brain. Creatively enough, termed as [the gut brain axis](https://fivethirtyeight.com/features/gut-week-gut-brain-axis-can-fixing-my-stomach-fix-me/), it is the sole reason for all the food and head related things. This system, largely involving the Vagus nerve, controls the body’s unconscious actions, such as digestion, excretion, and sexual arousal.

In the absence of certain (good) microbes, due to imbalanced diet (read junk eating), the neuroactive compounds, that regulate the above said unconscious actions, are profoundly altered. It turns out, what you eat changes the gut ecosystem, in turn resulting in altered vagal activity.
_**But, what happens in Vagus does not stay in Vagus!!**_
The altered vagal activity leads to a lot of intermittent chain reactions, including decreased pancreatic enzyme secretion, poor gallbladder function, overall disturbed gut function. This suppresses the intestinal immune system, decreases intestinal blood flow, increases intestinal permeability, eventually leading to leaky gut, causing digestive problems, inflammation and infection, further aggravating the issues.
The cytokines produced during this imbalance, cross the blood-brain barrier, leading to decreased activity in brain, decreased activation of the vagal motor nuclei, inflammation and decrease in nerve conductance. The effects being anything from mild headaches to aberrant stress to long term depression. A causal association, between gut microbes and cognitive decline and disorders of cognitive function such as Alzheimer's disease and multi-infarct dementia (high prevalence after the age of 65 years), is already been studied.
Wait a minute, before, you thought, when you are in stress you eat more, or when you are tensed you have gastric problems, or when you are hungry for long duration you get a headache. Now you understand that these things are connected, but, possibly in the opposite order, where the food you eat cause the stress, gastric issues and headache.

So, coming back the full circle, to where the story began...
The gut microbes (or the whole microbiome) play a very important role in your growth, wellbeing and longevity, from your birth to senility. In fact, it has been established that post-birth, the gut microbial colonization occurs in parallel with cognitive development. There is [increasing evidence](https://www.ncbi.nlm.nih.gov/pubmed/20966022) to support the view that the evolving cognitive activity is critically dependent on the microbiota and its metabolic activity.

_**Now that’s a lot of pondering!!**_
(as I said, that’s what we do best, right?)
So, the next time you think about a diet plan (especially the new year ones), or any resolution for that matter, [listen to your gut](https://bugspeaks.com/) and gradually change and adapt, without hitting the Vagus.
Or else, come and read this blog, again, next year!!
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Anxiety, Depression & Gut Microbiome
Author: BugSpeaks
Published: 2019-01-21
Category: Microbiome and Lifestyle
Meta Title: Anxiety, Depression & Gut Microbiome
Meta Description: Have you ever felt butterflies in your stomach, trembling hands and voice, sweaty palms, racing heart beat and heavy breathing&hel
URL: https://www.bugspeaks.com/blog/anxiety,-depression-&-gut-microbiome
Have you ever felt butterflies in your stomach, trembling hands and voice, sweaty palms, racing heart beat and heavy breathing… and you feel just fine briefly after? sounds familiar?
At some point in our lives, everyone has experienced this; when teacher asked us to solve a math problem in front of the whole class, may be while performing on stage or while talking in front of a large crowd. These manifestations mostly arise out of dreaded situations and these outcomes you feel is cumulatively called as “Anxiety” and specifically these are temporary in nature and hence termed as “Occasional Anxiety”.
But, if you scan through scientific literature and articles, anxiety is almost always coupled with depression, and commonly used together.
Anxiety is a type of emotion or feeling wherein [hypervigilance, attentional bias](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4211931/) and nervousness conquer the person. Whereas, depression is an observable trait, significantly affecting daily routines and moods of the individual, how they feel and think and even have some long-term effects. Like occasional anxiety, temporary episodes or moderate intensity of depression are termed as “Temporary Depression”. But care should be taken not to confuse this with ‘Anxiety and Depressive Disorders’, whose symptoms can persist for longer time with greater tendency to become a full-blown disorder.
Until recently, all these mental health and neurological disorders, specifically Anxiety and Depressive Disorders, have largely been understood in a unidirectional way as a sole function of brain and its nervous system. But imagine something sneaky at play here, something subtler than brain, yet having a greater impact than brain.
_Enter “Gut Microbiota”_
**The old friends and the Gut Brain Axis**
Rook et. al. proposed the ["old friends hypothesis](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5102282/)”, as an extension of the famous [hygiene hypothesis,](https://en.wikipedia.org/wiki/Hygiene_hypothesis) which states that we humans have evolved with a bunch of friendly and non-friendly microorganisms, since thousands of years (as species) and from our birth (as individuals). This is specifically true with respect to our guts, where these microorganisms have thrived as an entire ecosystem with continuous availability of nutrition. Current estimates indicate that the _human gut is occupied with approximately 100 trillion cells_ of different microorganisms, happily living within us.
The same _GI tract is also intertwined with ~600 million nerves of ENS_ (Enteric Nervous System), which works along with CNS (Central Nervous System) bidirectionally, controlling various digestive functions. Until recently, this bidirectional crosstalk, _known as "Gut Brain Axis" (GBA)_, was thought to maintain only the gastrointestinal homeostasis, some immune activation, enteric reflux and enteroendocrine signalling. A detailed account of this complex nervous system has been described in one of our other blogs “[The Brain in Your Gut](https://www.bugspeaks.com/blog-details?id=44&&title=The_brain_in_your_gut)”
However, peaked interest in the gut microbiota and its sheer number in the gut (I mean, a 100 trillion is a lot!!), forced us to view the function of GBA under a different light. Which indeed led to our understanding that GBA is indulged in monitoring and connecting the emotional and cognitive parts of human brain to peripheral gut. Which in simpler terms says that “_there is a new meaning for gut feeling_”. (Know more [here](https://www.bugspeaks.com/blog-details?id=46&&title=Microbiome_and_the_Brain_Interactions) and [here](https://www.bugspeaks.com/blog-details?id=19&&title=What_happens_in_Vagus,_does_not_stay_in_Vagus!!))
_So, What’s the take home message here?_
_A 100 trillion microbial cells are constantly interacting with 600 million neurons, communicating and controlling everything from when you feel hungry to why you feel angry!!_
It is critical to note that this is a two-way interface, and the interplay can happen from “_microbiome-to-brain_” or “_from brain-to-microbiome_”, which was a key aspect that was ignored before.
[**Brain to Microbiome – HPA Axis**](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5987167/)
In its simplest form, it is _a stress response_.
[Interleukin-6 (IL-6) is a component of immune system that gets released due to stress](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2978751/), which reduces the expression of glucocorticoid receptors and over activates the hypothalamic pituitary adrenal (HPA) axis (a kind of brain and hormonal interaction). This ends up in over activating the inflammatory reactions leading to increased pro-inflammatory cytokines and stress-induced cortisone. Increased level of cortisone is a risk factor or is strongly associated with anxiety.
On the other hand, same stress can change your gut microbiota (composition and abundance).
Several intestinal microbes are capable of reducing anxiety and are largely considered beneficial to us. Predominantly, [_Lactobacillus rhamnosus_ and few more from the genus _Lactobacillus and Bifidobacterium,_](https://www.progressnp.com/article/psychobiotics-bacterial-hope-depression/) are specifically capable of this function, through their anti-inflammatory and anti-oxidant actions. These beneficial bacteria can negate the inflammatory effects caused by IL6 and in turn reduce anxiety. However, the same stress can reduce the abundance of such beneficial bacteria, in turn increasing the risk for anxiety.

**Microbiome to Brain – The [Leaky Gut](https://www.ncbi.nlm.nih.gov/pubmed/18283240)**
Stress can also change your microbiota (composition and abundance), activating other components of this complex system, “_inducing_” anxiety and/or depression.
Some intestinal bacteria produce toxins, known as [Lipopolysaccharide (LPS)](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5987167/), which disturb the intestinal environment and its permeability. The disruption of the intestinal barrier can lead to entry of these toxins in the blood, a phenomenon popularly known as “_leaky gut_”. Later, LPS can disrupt the blood-brain barrier (which is very hard to do), enter brain and modulate mood and behaviour by increasing the activity of amygdala in the brain.
If unchecked, this also allows for entry of other environmental toxins and harmful agents, which increases the pro-inflammatory cytokines and neurotransmitter norepinephrine, and many other factors, leading to development of depression.
Several such behavioural changes, with direct or indirect involvement of gut microbiota, has been reported and studied till date, some of which are detailed [here](https://www.bugspeaks.com/blog-details?id=20&&title=The_gut_microbiome_influence_the_function_of_brain_and_behaviors_in_humans) and [here](https://www.bugspeaks.com/blog-details?id=54&&title=Mind_altering_microbes).
**Psychobiotics**
Now that we know that this is a two-way streak and changes in gut microbiome can affect brain and its functions, several combinations of probiotics and prebiotics, either as solitary or as combined supplementations, has been tested, under the collective name of “[Psychobiotics](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5102282/)”. The ultimate goal is either to manipulate or to naturally have a gut microbiota, enriched with commensals that can maintain healthy neural, behavioural, cognitive and emotional functions.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Kefir - Bridging traditional dietary and current nutritional practices
Author: BugSpeaks
Published: 2019-01-18
Category: Diet and Supplements
Meta Title: Kefir - Bridging traditional dietary and current nutritional practices
Meta Description: Kefir is a friendly probiotic drink with slightly fizzy and tangy taste, a FANTASTIC fermented milk beverage originating from East
URL: https://www.bugspeaks.com/blog/kefir---bridging-traditional-dietary-and-current-nutritional-practices
Kefir is a friendly probiotic drink with slightly fizzy and tangy taste, a FANTASTIC fermented milk beverage originating from Eastern Europe and Southwest Asia. It looks like and tastes like thin yogurt, but far superior than others, as a probiotic. It is incredibly beneficial for digestion and gut health maintenance when consumed regularly. It is loaded with valuable vitamins and minerals and contains easily digestible complete proteins. People with lactose intolerance can eat kefir without problems, adding to its list of benefits.

Kefir can be prepared from any type of milk, cow, goat or sheep, coconut, rice or soy. It is made by adding kefir “grains” to milk. These are not grains in the conventional sense, but cultures of yeast and lactic acid bacteria. Over a period of 24 hours’ time, the microorganisms in the kefir grains multiply and ferment the sugars in the milk, turning it into kefir.
Even though Kefir has been widely recognized as a health drink with benefits, recent development in the field of probiotics, have raised enormous interest in Kefir, with researchers reporting a wide spectrum of progressive elements present in it benefits the person who is consuming it, as it is known for its anticarcinogenic, anti-inflammatory, and antipathogenic effects.
Unfortunately, misuse of the term probiotic has also become a common place and a major issue, with many products exploiting the term without meeting the requisite criteria. Consequently, there is a certain level of apprehension about these effects of Kefir, bringing it under the scrutiny. Can Kefir really show these effects in human, as most of the studies are done on mice and cell lines? If so then, what is the mechanism? What is the uniqueness of Kefir in terms of its bacterial composition?
Initially, some nutritional benchmarking has clearly identified the presence of beneficial components within Kefir (see picture below). “[Antimicrobial and healing activity of kefir and kefiran extract](https://www.ncbi.nlm.nih.gov/pubmed/15848295)” has also be studied early on, demonstrating highest activity against Streptococcus _pyogenes._ Further, Lactobacilli have been recognized as a major component of Kefir, which have GRAS (Generally Regarded As Safe) status. Of these, Lactobacillus _kefiri_ has been identified as the key player in Kefir that has already assessed many functional properties, [safety characterization and antimicrobial properties](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4052788/). However, parameters regarding safety of Kefir was imperative, before calling them probiotics.

Paul Cotter, of Teagasc Food Research Centre in Cork, Ireland, approached the issue to answer such questions associated with kefir, [employing tools of high-throughput sequencing to analyse the microbial composition of Kefir](http://msystems.asm.org/content/1/5/e00052-16). His team of researchers performed Shotgun metagenomic sequencing of the microbial population of Kefir. Along with identifying the composition, they also determined the systematic progression of the microbial communities during fermentation. They observed that, as the fermentation progressed, the bacterial diversity diminished, and species that dominate the population at the beginning are replaced by others, just hours after. For instance, _Lactobacillus_ _kefiranofaciens_ was the dominant bacterial species recorded to be in kefir during early stages of fermentations, but _Leuconostoc_ _mesenteroides_ became more prevalent during later stages. They could further establish the link between individual species present in kefir, flavour generating compounds, several genes and associated pathways. They identified genes that are responsible for some of the intestinal benefits seen in kefir-drinkers. Through metabolomic approach they could map the acidic taste of kefir to the presence of Acetobacter _pasteurianus_ and cheesy flavours to Lactobacillus _kefiranofaciens_. Additionally, they were able to change the flavour and taste of kefir by altering the ratio of these identified microbes.
Metagenomics, again, have proven to be a new way to look at bacteria, not only within hosts, but within commodities that have traditionally proven to possess health benefits. This whole new approach of analysis could in fact provide scientific insights and widen the consumer audience by imparting necessary knowledge to bridge the gap between traditional dietary & current nutritional practices.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Fermented Foods & Microbiome
Author: BugSpeaks
Published: 2019-01-17
Category: Microbiome
Meta Title: Fermented Foods & Microbiome
Meta Description: Since early history fermented foods have been consumed and have been an integral part of our diet. Fermented food gives The Broccoli conundrum – to eat or not to eat? Starting with, how to m
URL: https://www.bugspeaks.com/blog/vegetables-and-gut-microbiome
**The Broccoli conundrum – to eat or not to eat?**
Starting with, how to make children eat broccoli and then how to eat them correctly - raw or cooked or moderately cooked, then moving on to [studying all the health benefits](https://www.ncbi.nlm.nih.gov/pubmed/29513123), so much so that claims, such as “[broccoli is the only vegetable you need to eat to boost your health](https://www.independent.co.uk/life-style/food-and-drink/broccoli-vegetable-healthy-gut-immune-system-doctor-chatterjee-a8347261.html)”, have been made constantly.
On the other hand “[broccoli and other cruciferous vegetables having a dark side](https://www.kevinstock.io/health/health-dangers-of-cruciferous-vegetables/)”, with specific examples of how it can be [bad for your thyroid](https://healthyeating.sfgate.com/broccoli-bad-thyroid-9761.html) and other health risks of (at least) having (only) broccoli, have also come up.
Perhaps _“to eat broccoli or not to eat broccoli”_ has been the biggest conflict of the decade!!
To complicate things a bit more, recent peak in interest about our gut microbiota has added another dimension to this conflict.
May be to solve this conflict for once and for all?
Let’s figure it out, shall we?
**Diet play an important role in establishing and maintain a healthy gut**
We get exposed to microbes right at birth and they start to establish as our microbiome, more often as a unique fingerprint. In our gut, there are trillions of microbes – both beneficial and not so beneficial ones - together having a major impact on our health. There is a huge diversity within the gut microbiome, yet it is cumulatively unique to that individual.
In context of the blog, apart from various other factors, our [food, nutrition and diet play a very critical tole in establishment of our gut microbiota](https://www.bustle.com/p/11-foods-to-eat-for-a-healthy-gut-microbiome-7951955).
**Generally speaking,**
[Whole grains, beans, fruits and vegetables packed with nutrients, seed our gut with healthy bacteria and helps in maintaining gut diversity](https://foodrevolution.org/blog/best-foods-for-gut-health/). Eating a lot of green leafy vegetables and low-sugar fruits establish our gut with healthy and diverse microbes. Fibre-rich foods are essential for gut health. Beneficial microbes absorb vitamins to enhance immunity and decrease inflammation.

To understand this better, here are some specific classes of vegetables and how they help to establish and sustain a healthy microbiota of the gut.
**Resistant starch vegetables for gut microbiome**
High starch content of some foods has been known to have adverse effects, and consuming excessive starch can indeed lead to health problems.

[_However, all starch is not bad and its misconception that high starch is detrimental to health_](https://www.thehealthyhomeeconomist.com/best-resistant-starch-for-gut-health/).
A sub-group of starches, called resistant starch, has lately gained importance for its beneficial impact. Specifically, individuals with digestive problems, by slowly adding such foods in one’s diet, have responded with alleviated symptoms like reduced gas and discomfort, along with an overall improvement in their digestive capabilities. Largely, such foods are deemed helpful in IBS, colitis, some allergies cases and in autoimmune diseases.
The primary reason why resistant starches are important is because of their ability of enhance friendly bacteria in our colon. Resistant starches cannot be broken down by our gut (they ‘resist” digestion). Unlike other starches, instead of getting digested and being absorbed as glucose, it passes through the small intestine to the colon, where it gets converted into favourable, energy boosting, inflammation supressing “short-chain fatty acids (SCFAs)” by the intestinal bacteria. In turn, the process gives the beneficial bacteria an abundance of nutrients for their growth and sustenance.
**Foods rich in fibres and gut microbiome**
Similar two-way streak has been observed between fibre rich foods and gut microbiome. Fibre rich foods boost the growth of beneficial gut bacteria (mostly, _Bifidobacteria_). Largely, there are two types of fibres, viz., soluble and insoluble fibres. Soluble fibres help in lowering blood glucose levels and LDL cholesterol, whereas, insoluble fibres concentrate more on cleansing our digestive system. Radishes, leeks, Jerusalem artichokes, asparagus, jicama, carrots and many others belong to the class of fibre rich foods.
**Cruciferous vegetables for gut health**
**

**
This category of vegetables has been [estimated to possess high fibre content, and hence has similar mode actions and beneficial effects](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2728691/) as fibre rich foods, described above.
The most interesting aspect of cruciferous vegetables, that has set itself apart from rest of the veggie categories, is the presence of distinctive phytochemicals, such as glucosinolates.
Further, [Isothiocyanates (ITCs) are a group of bioactive compounds that are generated by the break-down of glucosinolates and are deemed to have many health benefits, including anticarcinogenic properties](https://www.ncbi.nlm.nih.gov/pubmed/?term=9865427). Various researchers have reported that, ITCs are potential chemoprotectors, they fight cancer and supress the neoplastic effects of various carcinogens at different organ sites. A detailed analysis of ITCs and glucosinolates, post consumption of dietary crucifers, have also yielded similar observations, adding support to the published reports.
_The most fascinating aspect here is the connection between these phytochemicals and our gut microbiome._
For dietary crucifers to be effective as anticarcinogenic, glucosinolates must get converted to ITCs. In plants, myrosinase enzyme is the enzyme responsible for this conversion. However, these myrosinases gets deactivated after cooking. And, here enters the gut microbiota!!
Certain groups of bacteria populating the human gut have myrosinase-like activity, which metabolizes glucosinolates to ITCs and hence re-instating the anticarcinogenic property of cruciferous veggies. Hence, when we consume cooked cruciferous vegetables, we depend on gut microbiota to convert glucosinolates to ITCs.
**Diversity in vegetables, not quantity or specific type, is key to good gut health**
The same studies that established this fascinating connection, also identified diverse bacterial species that can degrade glucosinolates. Similar observations have been made with digestion of fibre rich foods, resistant starches and many others. _These strongly indicates, that any health benefits that is conferred by these vegetables are not only dependent on the quantity and type of vegetables we consume but also depends on bacterial composition of our gut._
Further, it is a two-way streak, where eating more vegetables alters the bacterial composition of the gut, which in turn aids the digestion of these foods. A diet including many different types of vegetables (consisting of 30 or more different plant types each week) has been correlated with a much higher diversity in their gut microbiota. This pattern was noted irrespective of the type of diet consumed by the participant, greater bacterial diversity was recorded in both meat-eaters and vegans, so long as they consumed a large variety of vegetables and plant matter.
**So, should we eat broccoli or not?**
Let’s make a simple check list and see if broccoli fits the bill.

So, yes!!
You can eat broccoli
But remember
[“_Diversity in vegetables, not quantity or specific type, is key to good gut health”_](http://www.thegoodgut.org/eating-for-gut-health-variety-not-quantity-of-vegetables-is-key/)
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## On Prebiotics
Author: BugSpeaks
Published: 2019-01-14
Category: Diet and Supplements
Meta Title: On Prebiotics
Meta Description: Let’s begin with our birth... Mother’s breast milk contains rela
URL: https://www.bugspeaks.com/blog/on-prebiotics
**Let’s begin with our birth...**
Mother’s breast milk contains relatively high concentrations of small oligosaccharides (short chain of sugars) that are undigestible by new-borns. The role of these oligosaccharides in breast milk was not understood at first and in fact researchers were puzzled as to why breast milk would contain components that are unusable by the new-born. Only later did they found out that these [oligosaccharides were fuelling growth of beneficial bifidobacteria](https://books.google.co.in/books/about/Gut_Insight.html?id=EQcXAgAAQBAJ&redir_esc=y) found in new-born’s gut. This also explained why bifidobacteria is the most predominant in the guts of breast-fed babies.
**

**
_These oligosaccharides, along with many other components of food, now constitute a class of food and supplementation called “Prebiotics”_
**Technically speaking…**
Prebiotics are dietary substances that selectively promote proliferation and activity of beneficial bacteria indigenous to the colon. The concept, first developed by Gibson and Roberfoid in 1995, has been refined and redefined on several occasions. Prebiotics are currently defined as “[_selectively fermented ingredients that result in specific changes in the composition and activity of the GI microbiota thus conferring benefits upon host health_](https://www.ncbi.nlm.nih.gov/pubmed/?term=25545101)”.
Prebiotics alone can offer protection against a range of chronic diseases or conditions common in humans, like IBD, Type 2 Diabetes, Obesity, CRC etc., by preventing colonization of enteric pathogens. However, the most ideal and effective prebiotic would also be able to improve bowel function, metabolic potential, mineral bioavailability, immune modulation and most importantly support the growth of a diverse set of beneficial microbes in the gut.

**How does it work?**
In context, the most important effect of these [prebiotics and their fermented products is to selectively alter the taxonomic composition, abundance and activity of the colonic microflora](https://books.google.co.in/books?id=H6HAAgAAQBAJ&pg=PP1&lpg=PP1&dq=Probiotics+and+Prebiotics+in+Food,+Nutrition+and+Health+edited+by+Semih+Otle&source=bl&ots=-0PfnlUMtG&sig=ACfU3U0HcPETlrc4XGQW3uumJwGbUGZsbw&hl=en&sa=X&ved=2ahUKEwinh_L9q4PgAhVISX0KHa0-D2kQ6AEwAXoECAUQAQ#v=onepage&q=Probiotics%20and%20Prebiotics%20in%20Food%2C%20Nutrition%20and%20Health%20edited%20by%20Semih%20Otle&f=false), eventually conferring demonstrated health benefits. For example, prebiotic supplementation can beneficially enhance Bifidobacterium spp., the most dominant and beneficial flora of the gut. The beneficial effects of the these bifidobacteria is critically dependent on their viability and metabolic activity, which in turn is dependent on the presence of complex carbohydrates such as oligosaccharides and their metabolic products. Therefore, prebiotics are largely considered as bifidogenic factors and has been effectively utilized in diet applications.
Further, prebiotic food ingredients generally escape assimilation in the small intestine (because of their chemical structure are resistant to digestive enzymes) and upon reaching the colon gets fermented by the microbiota. There are two basic types of fermentations that take place in the gut viz., saccharolytic fermentation (of sugar/saccharide-based prebiotics) and proteolytic fermentation (or amino acid/protein/fibre-based prebiotics).
Either way, [the end products of such fermentation are largely short chain fatty acids, including acetate, propionate and butyrate](https://www.ncbi.nlm.nih.gov/pubmed/?term=27224877). These may further be metabolized systemically or locally to generate energy for us. However, they also play several other key roles that can promote heath, as depicted in the figure below.

**Great!!**
**So… where do I find these prebiotics?**
[Oligosaccharides have been proposed as the quintessential prebiotics that includes a whole set of short-chain carbohydrates](https://www.ncbi.nlm.nih.gov/pubmed/?term=19087441), mostly nondigestible by humans, but are great food sources for the gut microbes.
Most important and studied prebiotics includes [fructo-oligosaccharides (FOS), mannose-oligosaccharides (MOS), galacto-oligosaccharides (GOS) and inulin-type fructans](https://www.ncbi.nlm.nih.gov/pubmed/?term=25545101). The target bacterial groups are typically Bifidobacterium and Lactobacillus. The prebiotic potential of this oligos stems from their selective fermentation by Bifidobacterium spp. and to a lesser extent by Lactobacillus spp. in the colonic microflora.
[Fructan prebiotics such as inulin and FOS occur naturally in various foods including cereals, fruits and vegetables and so are ubiquitous in most diets](https://books.google.co.in/books?id=H6HAAgAAQBAJ&pg=PP1&lpg=PP1&dq=Probiotics+and+Prebiotics+in+Food,+Nutrition+and+Health+edited+by+Semih+Otle&source=bl&ots=-0PfnlUMtG&sig=ACfU3U0HcPETlrc4XGQW3uumJwGbUGZsbw&hl=en&sa=X&ved=2ahUKEwinh_L9q4PgAhVISX0KHa0-D2kQ6AEwAXoECAUQAQ#v=onepage&q=Probiotics%20and%20Prebiotics%20in%20Food%2C%20Nutrition%20and%20Health%20edited%20by%20Semih%20Otle&f=false). The genera that uses these oligos and which contribute the most to our health include _Ruminococcus bromii, Roseburia intestinalis, Eubacterium rectale_ and _Faecalibacterium prausnitzi,_ but there are many others that may benefit from these prebiotics. Much of the interest in these oligos are also because of the fact the more than 36000 plants worldwide contain FOS. And inulin and other fructans are commonly found in onions (2-6%), garlic (9-16%), leek (3-10%), banana (0.3-07%), asparagus (10-15%), Jerusalem artichokes (15-20%), chicory (13-20%) and even wheat (1-4%).

Dietary constituents other than carbohydrates conceivably could function as prebiotics. For instance, cocoa flavonols can increase the relative abundance of Bifidobacterium and Lactobacillus, by selectively suppressing potentially pathogenic bacteria of C. histolyticum group.
Also, it is important to note that because these prebiotics reach colon and only then gets utilized, depending on the type of prebiotics they might get concentrated and utilized in various sections of colon, as shown in the infographic below.

**Prospects of prebiotics**
Other than supplementation of prebiotics, either naturally as food or as processed supplementation, they are being utilized as “[Synbiotics](Link%20to%20BK;s%20various%20biotics%20blog)” in combination with probiotics (live cultured beneficial bacteria). Further, there are many other desirable attributes that can be encompassed into the approach of enhanced prebiotics. [Properties like highly selective fermentation, increased persistence through the colon, anti-adhesive properties, attenuation of virulence of pathogens and reduction of gas production](https://www.ncbi.nlm.nih.gov/pubmed/?term=19087441) etc., have been studied exclusively. Prebiotics are increasingly used in development of new food products, eg. drinks, yogurts, biscuits and table spreads. Even though many of the positive effects of prebiotic consumption are evident, many of these health claims require further research.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Research Services - A Crown of Thorns
Author: BugSpeaks
Published: 2019-01-04
Category: Pivot and Progress
Meta Title: Research Services - A Crown of Thorns
Meta Description: On completion of our first year as a start-up, our CEO Kumar wrote The collaborative spirit cannot be taught. It just happens. In the last blog you would have r
URL: https://www.bugspeaks.com/blog/the-collaborative-team-spirit
The collaborative spirit cannot be taught. It just happens.
In the last blog you would have read about our pivot towards the microbiome. This would not be possible without all members of our team coming together and developing an awesome product. How data and information flows from one team to another is vital for meaningful science. It is also very essential for a seamless experience. I wanted to introduce the dynamic team behind it.
**Computational Biology**
**DNA: The RAM guzzlers!!**
**RNA: Probably the most vociferous team in the office.**
No matter how many terabytes of RAM is provided they manage to consume it. RNA, DNA, short read, long read, no matter what the sample or technology they have a solution for it.
For the microbiome program they have developed a platform that can take any type of sequencing data, 16s, metagenomic or metatransciptomic data and process them into actionable outcomes.They have probably processed close to a petabyte of microbiome data to develop this robust system. Every month they bring down the computational run time and reduce the size of the data footprint. They have also developed a robust systems biology engine to discover the unknown.

**Microbiome & Nutrition**
**DNA: The biome experts!!**
**RNA: Probably the most combative team in the office.**
They claim to know what every living organism on this planet feeds on. They know how bacteria, virus & fungus thrive.

For the microbiome program they have developed proprietary databases on nutrition, microbiome and human diseases. The team has spent countless hours in validating the correlation as well as causation between the microbiome and specific traits or diseases.
**Reporting & Content**
**DNA: The translation specialists.**
**RNA: They are the nimblest team in the organisation.**
Given any complex data, they will simplify it to a language understood by all.
For the microbiome program they have meticulously refined data delivery. The sliced the reports into multiple formats to better serve the consumers, the clinicians, the nutritionist and lifestyle coaches. Each report is handcrafted and personalized.

**Product Design**
**DNA: Wood is the new cardboard!!**
**RNA: They are the pickiest team.**
Their moon shot is handsfree faecal collection.
For the microbiome program they have hand crafted a beautiful kit that signifies how precious faecal matter is. With every iteration a new feature is introduced to make the collection to report experience seamless.

**The X Factor**
**DNA: The secret sauce**
**RNA: They are very silent, yet salient group.**
They make Bug Speaks talk. Even sing.
For the microbiome program they have taken raw data from.....

**AI**
**DNA: The horse whisperers.**
**RNA: They are the most secretive team.**
They talk to the data and some say the data talks back to them.
For the microbiome program they have implemented machine learning over knowledge graphs. They have also used blockchains to maintain transparency.

**Accounts & Logistics**
**DNA: The poop importers!!**
**RNA: They are busiest team in the office.**
“_Hello FedEx, we would like to import poop”._
_

_
For the microbiome program they have amalgamated multiple logistics partners to ensure there are no difficulty in sample transportation, no matter which part of the world the sample is coming from. They have also ensured that EMIs are available for all tests.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The Microbiome Pivot
Author: BugSpeaks
Published: 2019-01-01
Category: Pivot and Progress
Meta Title: The Microbiome Pivot
Meta Description: What drives Leucine Rich Bio? What is it that we really want to do?
Were the questions that kept coming back to me…<
URL: https://www.bugspeaks.com/blog/the-microbiome-pivot
What drives Leucine Rich Bio? What is it that we really want to do?
Were the questions that kept coming back to me…
And the answer always boiled down to healthcare.
_“Health care” and not “Sick care”_.
When we started the company, we wanted to provide the necessary answers to solve healthcare problems. We developed databases and algorithms. We crunched data and simplified complex interactions to meaningful actionable points. All this led to our expanded capabilities of looking at complex problems in biology and we did not limit ourselves to healthcare alone. Over 4 years we processed, analysed and reported over 100 terabytes of data for customers from 10 different countries.
And then came the pivot!!

I was nervous. I had to go up to my colleagues and tell them about the pivot. How we were going to stop all other projects and concentrate on “_one wellness product_”.
Why?
Because deep down all of us cared about human health. We cared about staying healthy and when it came to human health our passion produced creativity. But I was not sure whether my colleagues sensed my inner passion. I was nervous.
I gathered everyone.
I knew we would fail if everyone did not participate enthusiastically.
“_Let’s pivot around the microbiome_”, I said.
The room was silent with few glances at each other.
Team Leucine Rich Bio is known for many things, silence is not one of them. Raucous laughter with vociferous discussions is how team meetings usually go. Ask the people in the next building. This time was different though!!
“What’s pivot?”, asked one.
That’s when it dawned to me that I was talking in ‘_start-up lingo_’ to a bunch of ‘_scientists and techies_’. My 1-minute monologue on pivot was met with bombardment of ideas and solutions. We were going to pivot around the microbiome and all of us were backing it. All 22 of us. (More about this collaborative spirit in my next blog).
This was a great moment for us. We were not going to just churn data, we were going to have positive impact on everyone’s health.
Our health is impacted greatly by the microbiome. The human microbiome is defined as the collective genomes of the microbes (bacteria, bacteriophage, fungi, protozoa and viruses) that live inside our body. In simple words it’s the different kind of microbes living inside and on our body. Some estimates suggest that there are more microbes in our body than human cells. Given the sheer volume of the microbiome, it must have a significant influence on our body.
Of these, 90% of the microbes reside in our gut. The gut microflora is influenced by nutrition and lifestyle. So, in essence, what we eat and how we live shapes our microbiome, which in turn influences our health.
Below is a complex graph showing the different species of microbes found in different parts our body.

We had started working on the microbiome in 2015. The very first sample we analyzed looked at a few hundred microbes. We now look at around 1,40,000 microbes. We have identified close to 10,000 different species of microbes. Usually we see anywhere from 800 to 2000 different types of microbes in an individual.
I’ll let you read that sentence again.
“800 to 2000 different types of microbes are present in an individual’s gut”.
Each of them have millions of copies. Just fathom the impact that it might have on us. This impact has been shown by different academic labs around the world. As we delved into the science, we realized the impact is even greater.
_To top it off, the solution is simple. Nutrition._
Our mission from the beginning was to bring about a paradigm shift to _Health Care from Sick Care_. We strongly believe the microbiome is the way forward to achieve our mission and the technology that we have developed is on par if not better than the rest.
We pivoted around the microbiome so that we could leverage our expertise and passion to provide everyone with better health. Better health is not a moonshot, it is as simple as including an extra food item in your diet.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Studies Suggest the Relationship Between the Gut Microbiome and Allergic Diseases
Author: BugSpeaks
Published: 2018-07-27
Category: Microbiome and Disease
Meta Title: Studies Suggest the Relationship Between the Gut Microbiome and Allergic Diseases
Meta Description: The past few years have witnessed the increased cases of patients suffering from allergies and inflammatory diseases. Scientific explorations have sug
URL: https://www.bugspeaks.com/blog/studies-suggest-the-relationship-between-the-gut-microbiome-and-allergic-diseases
The past few years have witnessed the increased cases of patients suffering from allergies and inflammatory diseases. Scientific explorations have suggested the existence of the relationship between the gut microbiome and allergic diseases. The gut microbiome produces both beneficial and damaging metabolites from the diet. Along with bacterial components, these metabolites modulate the host immunity, and significant influence on the occurrence of allergic illnesses.
The human gut harbors several trillions of commensal microorganisms, mainly the bacterial population with more than thousands of species. This collection of microbes is generally represented as the gut microbiome. Due to obvious reasons, culture-based analysis of microbiome diversity is replaced by the recent advanced high-throughput DNA sequencing approaches, such as the bacterial 16S ribosomal RNA sequencing and microbiomics. These approaches enable the correct documentation of commensal bacteria directly without requiring to culture. Such studies have evidenced that alteration in the gut microflora composition or declined diversity, also known as dysbiosis state may lead to the development of intestinal inflammations and subsequently cause allergic conditions. For example, in a study comprising patients having food allergies in the United States has revealed the occurrence of reduced microbial diversity in the gut, and found an increased number of bacterial species belonging to Bacteroidales, while Clostridiales were found in low numbers. Further, some gut bacteria produce useful metabolites using the host's diet, and they directly take part in the mediation of host’s immune responses. Researchers have highlighted the role of commensal gut bacteria and their metabolites in regulating host immune responses and allergic diseases.

Long-chain fatty acids (LCFAs), such as ω3 and ω6 fatty acids function as energy sources and take part in the immune responses. However, mammals cannot produce LCFAs, and interestingly they are produced by the gut microbes using dietary components. Further, it is suggested that ω3 fatty acids have anti-inflammatory and anti-allergic properties (Miyata and Arita 2015). The gut commensal bacteria, mainly lactic acid bacteria (e.g., Lactobacillus plantarum) are reported to produce ω6 fatty acid-derived lipid metabolites (linoleic acids, oxo and hydroxy fatty acids). Likewise, 17,18-epoxy-eicosatetraenoic acid, an anti-allergic lipid metabolite is speculated to be produced in the colon via cytochrome P450 function. However, evidences have suggested the role of commensal bacteria in LCFAs metabolism. In a study, germ-free animals were shown to produce high levels of colonic lipid metabolites, such as 17-hydroxy docosahexaenoic acid, 14-hydroxy docosahexaenoic acid, resolvin D1, and protectin D1 when compared to conventional mice. Resolvin D1 is known to down-regulate the expression of interleukin-1β (IL-1β) during microbial infections. Further, IL-1β intensifies allergic reactions to cause asthma, atopic eczema, and dermatitis. Also, it is suggested that microbe-dependent inhibition of resolvin D1 production is linked to allergic inflammations (Hirata and Kunisawa 2017). Experimental evidences suggest that 10-hydroxy-cis-12-octadecenoic acid produced by Lactobacillus spp. may prevent the progress of food allergies, which is mediated by maintaining intestinal epithelial barrier function. Figure 1 illustrates the role of bacterial metabolites in the gut towards regulating immunity for the allergic diseases.
[](/media/uploads/blog/163a.jpg)
[

](/media/uploads/blog/163a.jpg)
**Figure-1: Bacterial metabolites in the gut regulate immune responses for the allergic diseases. Bacterial metabolites regulate versatile biologic and immunologic functions related to allergic diseases. Epithelial barrier function is enhanced by 10-hydroxy-cis-12-octadecenoic acid, a linoleic acid-derived metabolite, and SCFAs such acetate and butylate, fermentation products by some bacteria. In addition, SCFAs (e.g., acetate, butyrate, and propionate) as well as D-type tryptophan have a potential to enhance the induction of Treg cells. Vitamin B9 plays a key role in the maintenance of Treg cells and its metabolite prevents the activation of MAIT cells by competing with vitamin B2 metabolite, MAIT cell activating ligand, in their binding to MR1. PPAR ligands such as LCFAs decrease the inflammatory cytokines such as IL-8 and induce the production of anti-inflammatory cytokines such as IL-10. (Source: Hirata and Kunisawa 2017; [https://doi.org/10.1016/j.alit.2017.06.008](https://doi.org/10.1016/j.alit.2017.06.008))**
In a recent cohort study, the association amongst the gut microbiome, their lipids, and allergic diseases in humans was established. It was observed that the composition of the gut microbiota plays a significant role in the development of atopy and asthma in childhood. 16S rRNA sequencing analysis evidenced that high risk for the development of allergy in neonates is due to the reduced abundance of gut bacterial species, such as Bifidobacterium, Akkermansia and Faecalibacterium and increased abundance of fungi, such as Candida and Rhodotorula. Interestingly, the low-risk subjects were observed to have increased levels of anti-inflammatory lipid metabolites (docosapentaenoate and dihomo-γ-linolenate). On the other hand, high-risk groups were shown with enhanced levels of pro-inflammatory metabolites, such as 12,13-dihydroxy-9Z-octadecenoic acid, stigmasterol and sitosterol, and 8-hydoxyoctanoate (Fujimura et al. 2016). LPSAs are reported to modulate immune reactions via the peroxisome proliferator-activated receptor (PPAR) family, i.e., the most common negative regulators for allergic conditions. As stated by Hirata and Kunisawa (2017), Bacteroides thetaiotaomicron stimulates PPAR-γ-dependent export of NF-κB from the nucleus, and subsequently it decreases NF-κB-dependent IL-8 production to curb the IL-8-intermediated infiltration of granulocytes in bronchial allergy.
Moreover, immune cells, including iNKT (invariant natural killer T) cells are activated by microbial glycolipids. For example, Sphingomonas spp. producing glycosphingolipids, Sphingomonas spp., and Borrelia burgdorferi producing glycodiacylglycerols, Helicobacter pylori producing cholesteryl-α-glucoside. Further, D-type amino acids (e.g., D-alanine, D-glutamic acid, D-asparagine, and D-prorine) secreted from the gut bacteria are believed to control host allergic reactions. Likewise, D-tryptophan from the probiotic bacteria, such as Lactobacillus rhamnosus GG, Lactobacillus casei W56 is shown to ameliorate allergic airway inflammation and hyperresponsiveness in the gut and lung. Similarly, microbial vitamins, including B-family members and K, serve as an important supplementary source of vitamins that are take part in the regulation of the immune responses and controlling allergic diseases. Likewise, Short-chain fatty acids, such as propionate (produced from Bacteroidetes and some Firmicutes), acetate (produced from many genera of gut microflora, including Bifidobacterium spp.), butyrate (produced from Clostridium cluster XIVa species and Bacteroides thetaiotaomicron) exert diverse anti-allergic properties.
Overall, commensal gut bacteria produce many useful metabolites that are involved in regulating allergic responses mediated via various ways, such as the induction of Treg cells, up-regulation of IL-10 expression, suppression of the Th2-type phenotype and maintenance of the gut barrier function. Also, they are known to produce pro-inflammatory metabolites. There are many factors that are involved in the regulation of immune response and allergic diseases. Hence, having a right balance between diet and the gut microbiome may certainly benefit in overcoming allergic diseases. However, more research is highly recommended in this regard to clearly understand about the mechanisms involved and to disclose various cross-talk between commensal microbes in the gut and host immune system.
**Keywords:** The gut microbes, metabolites, microbiome, short-chain fatty acids, vitamins, allergic diseases, immunity
**References:**
* Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, Wegienka G. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nature medicine. 2016 Oct;22(10):1187.
* Hirata SI, Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergology International. 2017;66(4):523-8.
* Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergology International. 2015;64(1):27-34.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The Gut Microbiome Alterations is related to Autism Spectrum Disorders
Author: BugSpeaks
Published: 2018-07-17
Category: Gestational and Neonatal
Meta Title: The Gut Microbiome Alterations is related to Autism Spectrum Disorders
Meta Description: Autism spectrum disorder (ASD) is a hereditary linked disorder of brain function with symptoms, such as restricted forms of behaviors and activities,
URL: https://www.bugspeaks.com/blog/the-gut-microbiome-alterations-is-related-to-autism-spectrum-disorders
Autism spectrum disorder (ASD) is a hereditary linked disorder of brain function with symptoms, such as restricted forms of behaviors and activities, or persistent discrepancies in societal interaction and communication. ASD has a significant impact on the growth and development of children and on the general public. The assessed incidence of ASD in 2012 was about 14.6 per 1,000 children (aged 8 years), and its occurrence was relatively higher in boys (23.6 per 1,000) when compared to girls (5.3 per 1,000). As per the estimate, in the United States alone, the cost of caring for a child with ASD was about $1.4 million, while in the United Kingdom, it was reported to be £0.92 million (Li et al. 2017). The main costs associated with the care of children with ASD are special education services and a loss of parental productivity. Thus, the financial effects of ASD have driven scientists to hunt for effective medications (Buescher et al. 2014; Li et al. 2017).
ASD is described by numerous clinical endophenotypes, and is hypothetically connected with certain comorbidities. As per the current recommendations, gastrointestinal symptoms are the reasons for the comorbidity in patients with ASD, however underlying mechanisms are unfamiliar. Several investigations have revealed that patients with ASD have alterations in the fecal flora composition, and the gut microbiome metabolic products. The dietary constituents along with metabolic activities of the microbiome are now assumed to be linked to alterations in behavior and cognition, mainly in patients having neurodegenerative diseases. The gut microbiome have a significant role in the development of the brain and controls the behavior via the neuroimmune, neuroendocrine, and autonomic nervous systems. There is a bidirectional communication between the central nervous system and the gastrointestinal tract (brain-gut axis) and the gut microbiome functions. As stated by most recent studies, collective pathogenetic elements and pathophysiological mechanisms probably associating to gastrointestinal disturbances and ASD include intestinal inflammation with or without autoimmunity, gluten-related disorders (celiac disease, wheat allergy, non-celiac gluten sensitivity), immunoglobulin E-mediated and/or cell-mediated gastrointestinal food allergies, abdominal pain, dysautonomia connected with gastroesophageal reflux and gastrointestinal dysmotility. In addition, dysregulation of the gut microbiome has the capability to upset intestinal permeability, motility and sensitivity, and mucosal immune function.
The human gut contains about 1000 grams of bacteria, and approximately about 9.9 million numbers of bacterial genes exist in the gut i.e., the ratio of microbiome DNA and host DNA is 10:1. Gastrointestinal indications are noticeable in ASD patients. Researchers have observed more gastrointestinal syndromes, including diarrhea and constipation in children having ASD compared with their unaffected siblings. ASD patients with gastrointestinal syndromes display substantial interactive or behavioral manifestations, including self-injury, aggression and anxiety. Many studies have demonstrated that the gut microbiota composition and function is directly or indirectly linked with ASD signs, partly by prompting the immune system and metabolic rate. Patients with ASD are observed to have a higher percentage of abnormal intestinal permeability leading to greater antigenic load from the gastrointestinal tract. ASD-associated cytokines, such as interferon-γ (IFN-γ), interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) in addition to lymphocytes present in the circulation cross the blood-brain barrier (BBB). Successively, IL-6, IL-1β and TNF-α bind to brain endothelial cells and encourage immune responses in the brain. Variations in the gut microbiota composition and their metabolic products secretion are generally noticed in ASD patients and in animal models of ASD. In a mouse model study featuring ASD, researchers have found that gastrointestinal barrier defects caused microbiota alterations. They observed more abundant bacteria, belonging to Prevotellaceae, Porphyromonadaceae, unclassified Bacteroidales and Lachnospiraceae in offspring of mothers with maternal immune activation, while in control offspring, Erysipelotrichaceae, Ruminococcaceae and Alcaligenaceae were found to occur more abundantly. The gut microbiota diversity in children with ASD is observed to be very less as compared to that of children without ASD. In ASD patients, higher levels of Desulfovibrio, Lactobacillus, Bacteroidetes, Clostridium, Caloramator and Sarcina, and lower levels of Bifidobacterium and Firmicutes are observed. Further, children with ASD and gastrointestinal syndromes possess inferior loads of unclassified Veillonellaceae and the genera, Coprococcus, and Prevotella than that found in gastrointestinal syndrome-free neurotypical children. Clostridium histolyticum, found in the fecal samples of children with ASD was at a higher level when compared with healthy children without ASD. Moreover, altered levels of Prevotella, Bifidobacterium, and Sutterella are found in children with ASD. Researchers also have observed that Candida species (Candida krusei and C. glabrata) were 2-times higher in individuals affected with autism than in normal individuals. Also, it is observed that Candida species can release ammonia and toxins that can encourage autistic behaviors.
The gut–brain axis is viewed as a way of interaction between the gut microbiome and the brain, and it is a two-way communication structure. The outcome of several studies suggests that the gut-brain axis contributes to the origin and development of ASD. In a comprehensive review by Li et al. (2017) stated that the gut flora affects functions of the brain via the neuroendocrine, neuroimmune and autonomic nervous systems and through toxin production by microbiota (Figure 1). The presence of millions of neurons in the mucosa of the gastrointestinal tract constitutes the enteric nervous system and modulate various gastrointestinal functions. Hence, the gut is recognized as ‘the second brain’. ?The potential interactions between the gut microbiota and the pathogenesis of ASD is outlined in Figure 1.

[](/media/uploads/blog/162a.jpg)
[

](/media/uploads/blog/162a.jpg)
**Figure-1: Potential relationships between the microbiota and ASD (the gut-brain axis). The production of metabolites, such as SCFAs and toxin metabolites, by certain microbiota (e.g., Lactobacillus) can cross the “leaky gut” to affect brain function. Some microbiota can produce neuroactive compounds (e.g., 5-HT and GABA) that cross the “leaky gut” and influence brain function and induce abnormal behaviors. These neuroactive compounds can directly influence the HPA axis and increase circulating levels of cortisol. Metabolites, certain microbiota and neuroactive compounds can activate enteric neurons and affect brain function through the vagus nerve. Some microbiota and metabolites can activate gut immune cells, which can release cytokines into circulation. 4-EPS, 4-ethylphenyl sulfate; 5-HT, serotonin; HPA, hypothalamic–pituitary–adrenal; SCFAs, short-chain fatty acids; BBB, blood-brain barrier; 5-HT, 5-hydroxytryptamine; ENS, enteric nervous system; GABA, γ-aminobutyric acid; DA, dopamine. (Adapted from Li et al. (2017); doi: [10.3389/fncel.2017.00120](https://dx.doi.org/10.3389%2Ffncel.2017.00120))**
The gut microbiota secretes certain metabolites, such as phenolics, short-chain fatty acids (acetic acid, proprionic acid, isobutyric acid, butyrate, valeric acid and isovaleric acid), and free amino acids that affect ASD-like behaviors via the vagal pathways mediating the microbiome-brain-gut axis communication. Likewise, the gut microbiota releases neuroactive compounds (dopamine, 5-HT, γ-aminobutyric acid, and histamine), which trigger or impede central neurons via the vagus nerve.
Currently, there are no available effective therapies for ASD. Parents often take their children to obtain mediations that are personalized to their specific needs. Recent evidences have indicated that the gut microbiota modulation can be a potential therapy in children having ASD. In this regard, prebiotics, probiotics, fecal microbiota transplantation and a suitable personalized diet have gained substantial attention in recent days. Moreover, microbiome-facilitated therapies are relatively safe and effective. Recent clinical studies have reported that ASD patient’s symptoms can be improved by treatments that normalize the gut microbiota. Nevertheless, more research studies involving more participants are required to know about the treatments that supports the gut microbiome in overcoming ASD symptoms.
**Keywords:**
The gut microbiota, microbiome, Autism spectrum disorder, Brain-gut axis, Probiotics, Fecal microbiota transplantation, Microbiome-mediated therapies
**References:**
* Li Q, Han Y, Dy AB, Hagerman RJ. The gut microbiota and autism spectrum disorders. Frontiers in cellular neuroscience. 2017 Apr 28;11:120.
* Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015 Jan 1;138:179-87.
* Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA pediatrics. 2014 Aug 1;168(8):721-8.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The gut microbiome have a role in Dementia
Author: BugSpeaks
Published: 2018-07-11
Category: Microbiome and Disease
Meta Title: The gut microbiome have a role in Dementia
Meta Description: Dementia, as a comprehensive type of brain disease is known to upset an individual’s daily activities. The disease is characterized by the deter
URL: https://www.bugspeaks.com/blog/the-gut-microbiome-have-a-role-in-dementia
Dementia, as a comprehensive type of brain disease is known to upset an individual’s daily activities. The disease is characterized by the deteriorated memory, thinking ability, behavior and the capability to carry out everyday functions. Globally, ~47.5 million individuals are affected and the prevalence of dementia is increasing continuously. Dementia leads to disability and dependency amongst the aged people, and thus has a colossal physical, mental, societal and economic effect on families, caregivers, and the general public. The common form of dementia is the Alzheimer’s disease, which account to about 60-70% of the cases followed by other forms, such as Lewy body dementia, vascular dementia, frontotemporal dementia, and Parkinson’s disease with dementia.
Recent research advancements have deciphered the role of gut microbiome and its composition associated with the gut permeability and inflammation in dementia patients. Also, it is hypothesized that dysbiosis (an impaired microbiota) may augment the gut permeability, microbial translocation and trigger an inflammatory-immune response, which may perhaps encourage pathogenesis and progression of dementia. In this regard, a novel approach is suggested for the management of these health disorders, and as an adjuvant for psychiatric treatment of neurological disorders, including dementia and other interrelated diseases via modulating the microbiota composition (e.g., with the use of probiotics).
The expansion of advanced high-throughput analytical techniques including next generation sequencing has disclosed the possible role of the commensal microbial population, particularly the gut microbiome in several human diseases. Lately, the theory of the gut brain-axis has been well-established. Accordingly, a communication exists between the gut and brain site to control and mediate the process of many biological pathways involving the autonomic and enteric nervous system, the neuroendocrine system and the immune system. However, any deviations in such communication due to dysbiosis may involve in disease development. For example, the enteric microbiota i.e., Bifidobacterium and Escherichia, which are considered as both commensal and pathogenic microbes are identified to have interactions between the brain and gut axis.
The gut microbiota supports numerous daily activities of the brain, comprising the regulation of the hypothalamic-pituitary-adrenal (HPA) axis activation state. The HPA axis activation releases cortisol, which in turn manage the triggering of microglia of the brain, and influence to release cytokines. This action rule the functions of the central nervous system through various communications, including vagal nerve and adrenergic nerve activation in addition to the secretion of many molecular candidates (endocrine hormones, neuropeptides, neurotransmitters, and immunomodulators). The stress hormones of hosts, for example noradrenaline might affect signalling between bacteria or their activities in the gut and lead to changes in the microbial diversity and overall functions of the gut microbiota. Nevertheless, these gut bacteria synthesize and release many neurotransmitters and neuromodulators, and induce the synthesis as well as the release of neuropeptides from enteroendocrine cells. For instance: the species of Bifidobacterium and Lactobacillus produce short-chain fatty acids; spore-forming bacteria produce 5-HT; Bacillus, Escherichia and Saccharomyces spp. secrete norepinephrine; Bacillus and Lactobacillus spp. produce dopamine and acetylcholine, respectively. In specific, recent findings have suggested that microglia maturation and function is governed by the short-chain fatty acids produced by the gut microbiota, and hence highlighted on the possible usefulness of the gut microbiome as an impending diagnostic target in dementia and its cure.
The gut microbiota regulates neuro-inflammations and the HPA axis activity, and might lead to Alzheimer’s disease. The neuropathological features of Alzheimer’s disease are the two pathologic protein aggregates, namely amyloid beta (Aβ) plaques and hyper-phosphorylated tangles of tau-protein, which cause neuro-inflammation, mainly facilitated by the innate immune system. This process mainly includes microglia cells that represent the resident macrophages of the brain. Though microglia cells can remove extracellular Aβ aggregates, in later phases of the disease, cells remain in a dystrophic state and fail to exert their positive actions. In addition, the inflammation and the pathogens (e.g., bacteria: Salmonella enterica; fungi: Candida albicans; viruses: herpes simplex virus; and parasites: Toxoplasma gondii) interactions can also lead to the development of Alzheimer’s disease. Thus, a strong gut microbiota may pay a significant role in preventing general infections by restricting pathogen growth, improving the microbial barriers, and enhancing the immune response. The representation of a few key factors in the Alzheimer’s disease pathogenesis is given in the figure-1.
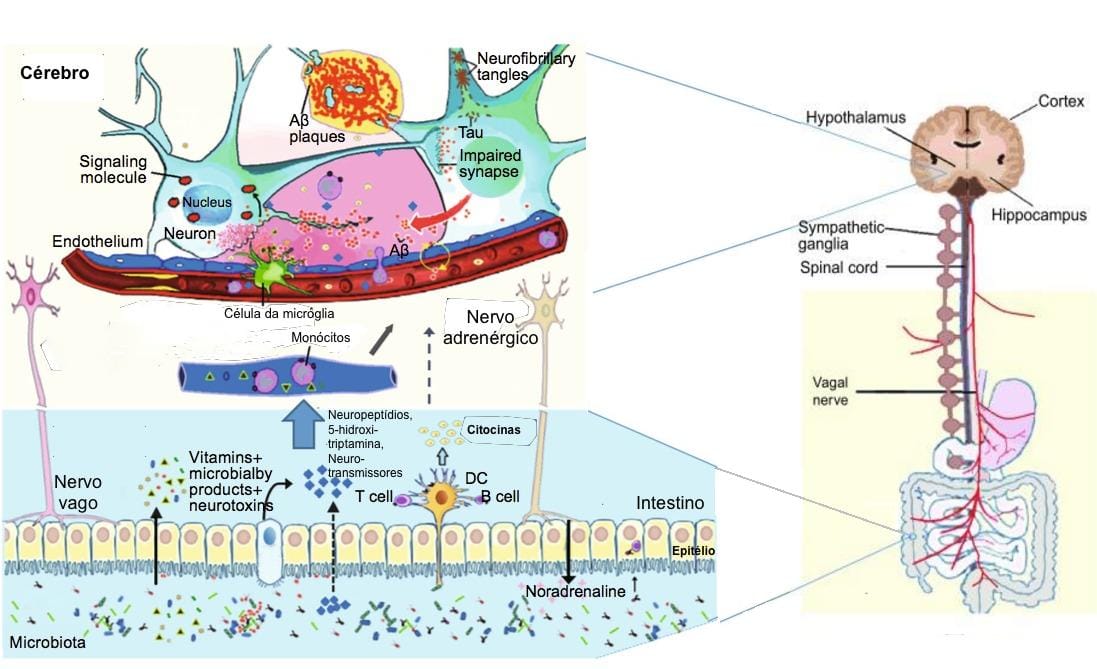
[](/media/uploads/blog/161a.jpg)
[

](/media/uploads/blog/161a.jpg)
**Figure -1: Schematic of some key players in the pathogenesis of AD. The gut microbiota regulation of neuro-inflammation and the hypothalamic–pituitary–adrenal (HPA) axis activity and may lead to AD. The bacterial products that gain access to the brain through the bloodstream and the area postrema, via cytokine release from mucosal immune cells, through the release of gut hormones such as 5-HT from EEC cells, or via afferent neural pathways, including the vagal nerve. NP: Neuropeptide; NT: Neurotransmitter; 5-HT: 5-hydroxytryptamine; DC: Dendritic cell; EEC: Enteroendocrine cell; Aβ: amyloid beta protein; AD: Alzheimer’s disease.**
**(Adapted from Alkasir et al. 2017; doi: [10.1007/s13238-016-0338-6](https://dx.doi.org/10.1007%2Fs13238-016-0338-6)).**
In the course of ageing, the gut microbiome composition undergoes fluctuations. In aged people, decreased microbial diversity with a loss of helpful taxa and increased facultative pathogens have been observed. Further, aging is connected with inflammation, which is also represented as inflammaging, which is linked with the accelerated gut permeability, mucosal inflammation and bacterial translocation. As aging is one of the chief risk factors of dementia, it is expected that the gut-brain axis is disapprovingly involved in the development of dementia. Considering this, a precise diet and the surrounding environment play a vital role in shaping the good microbiome composition and help in controlling many metabolic disorders. Some of the animal investigations have suggested that dementia is associated with fluctuations in the gut microbiome composition.
Studies have clearly indicated the positive link between the gut microbiome composition and the neuropathology of dementia. However, more investigations on the gut microflora is required to enable additional novel vision into the biology of dementia. This will facilitate in the development of novel therapeutic strategies for dementia. As stated in a review by Alkasir et al. (2017), the modulation of gut microbiota (by probiotics or other dietary intervention) or direct targeting of gut microbiota enzymes (by pharmacological inhibitors or activators) may be a growing area for pharmaceutical and functional food industries, with the goal of decreasing the widespread growth of adiposity, insulin resistance, Alzheimer’s disease, and other metabolic diseases.
**Keywords:**
Gut microbiome, Dysbiosis, Dementia, Alzheimer’s disease, Neuro-inflammation, Probiotics, Prebiotics
**References:**
* Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein & cell. 2017 Feb 1;8(2):90-102.
* Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci. 2016. doi: 10.1007/s11427-016-5083-9.
* Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutrition reviews. 2016 Oct 1;74(10):624-34.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The gut microbiome promotes immune homeostasis
Author: BugSpeaks
Published: 2018-07-06
Category: Microbiome and Disease
Meta Title: The gut microbiome promotes immune homeostasis
Meta Description: The gut microbiome is defined as the group of microorganisms and their genes existing inside the human gut. Recently, it has arisen as a primary facto
URL: https://www.bugspeaks.com/blog/the-gut-microbiome-promotes-immune-homeostasis
The gut microbiome is defined as the group of microorganisms and their genes existing inside the human gut. Recently, it has arisen as a primary factor in various human health conditions and diseases. A symbiotic relationship between humans and microbes is prevailing ever since from the birth, and any perturbations in this relationship could be the basis for immunological dysregulation, which might lead to the onset of many health complications and diseases including inflammatory bowel disease, autoimmunity, rheumatoid arthritis, cancer and multiple sclerosis. Symbiotic bacteria of the human gut provide a good environment and several advantages, such as providing essential nutrients, metabolizing indigestible food compounds, defense against the opportunistic pathogen colonization, and even contribute in the development of the intestinal architecture.
The immune system is responsible to recognize, retort and acclimatize to numerous foreign substances/molecules and also self-molecules. Thus, immune responses significantly modulate the development of health and disease. The human gut placidly co-exist with vast and diverse beneficial microbiota, and our immune system has evolved with gut bacteria to protect against various infectious microbial pathogens. In specific, the gastrointestinal tract is the main site at which both symbiotic and pathogenic microbes interact with the host immune system. More recent evidences have suggested a beneficial partnership has evolved amongst the symbiotic gut bacteria and the host immune system. The molecular communications/exchanges between them are directly linked to the growth of immune responses, and sequentially, the immune system of the host shapes the configuration of the microbiota.
Research evidences have related the role of the gut microbiota in shaping our immune system responses during diseases, however, the question still remains as to which specific bacteria are responsible in the mediation of these helpful responses and, more importantly, how this is accomplished. Some inspiring examples of the gut microorganisms having a pivotal role in the prevention of inflammatory bowel diseases, and beneficial immune reactions they stimulate during the defense are highlighted below.
At the start of 1900s, Ilya Mechnikov proposed for the first time about the use of live microbes to sustain bowel health and prolong life. Presently, the word “probiotic” is used for describing dietetic microbes with the ability of offering health benefits to the host. Numerous bacterial species either individually or in combination have been reported to ameliorate the symptoms of inflammatory bowel diseases in humans and mouse models. Some of them include Bifidobacteria lactis, B. infantis, E. coli Nissle 1917, Lactobacillus rhamnosus GG, L. salivarius, L. fermentum, Bacteriodes fragilis, B. thetaiotaomicron, etc. Mainly, these probiotic strains decrease toxic microbial metabolic events, however, more recent evidences have demonstrated their capability to modulate gut immune responses. The probiotic strains have a common feature of controlling the inflammation. The probiotic bacteria act on different cell types including epithelial cells, T cells and dendritic cells. Further, evidences have suggested that these probiotic strains induce regulatory T cells, which is the central to regulating inflammation and diseases. For example, treating colitic mice with the VSL#3 (a mixture of 8 lactic acid bacteria probiotic strains) increased the production of interleukin (IL-10) and the percentage of TGFβ-expressing T cells. Likewise, in another model of pathogen-induced inflammation study, the treatment of mice with B. infantis significantly down-regulated the intestinal inflammation and increased the number of CD4+CD25+ TReg cells. Moreover, the adaptive transmission of the CD4+CD25+ TReg-cells from mice served with B. infantis repressed inflammation-related activation of NF-κB (nuclear factor-κB) in the recipient mice. Interestingly, when the bone-marrow-derived dendritic cells incubated with L. rhamnosus were transferred into a colitic mice, a protection against inflammation and disease was observed. Further studies have shown that L. rhamnosus-treated dendritic cells can initiate TReg-cell activity. It has been shown that certain patients with Crohn's disease possess a reduced level of a noticeable gut bacteria, Faecalibacterium prausnitzii. As reviewed and explained by Round and Mazmanian (2009), this organism or its secreted substances are capable of inducing IL-10 expression (anti-inflammatory response), and ameliorate the induction of tumor necrosis factor-α (TNF-α) and bowel diseases, when administered orally to the experimental animals. This study further indicated that there exists a direct link between the reduced numbers of F. prausnitzii from the gut microbiota and the development of a bowel disease. This clearly indicates that symbiotic gut bacteria can intermediate in inflammatory bowel diseases and health. However, the explicit molecules/compounds secreted by these beneficial microbes of gut microbiota to guide in the immune responses still remains unclear. But, existing scientific data support the clue that symbiotic gut microbes actively interconnect with the host immune system to modulate anti-inflammatory processes.
A single molecule, polysaccharide A (PSA) secreted by the human commensal bacterium (Bacteroides fragilis) is predicted to shape favorable immune responses (Figure 1). When the germ-free mice were colonized with B. fragilis or treated with the purified PSA, the cellular and physical development of the immune system was improved with the increase of differentiated splenic CD4+ T cells. Experimental evidences have suggested that PSA protects by decreasing the levels of the pro-inflammatory cytokines (TNFα, IL-17 and IL-23). Also, it inhibits epithelial hyperplasia and neutrophil infiltration to the gut associated with disease induction in these models (Round and Mazmanian 2009). Likewise, the gut microbiota on T-cells immune responses are depicted in figure 1.

[](/media/uploads/blog/160a.jpg)
[

](/media/uploads/blog/160a.jpg)
**Figure-1: Impact of the gut microbiota on T-cells immune responses. Colonization with segmented filamentous bacteria (SFB) occurs by intimate attachment to the intestinal epithelium and promotes the development of T-helper 17 (Th17) cells via intestinal epithelial cell (IEC)-derived cytokines, serum amyloid A (SAA), as well as antigen presentation by dendritic cells (DCs). Adhesion of SFB to IEC can potentially generate a circuit, wherein DC-derived IL-23 stimulates IL-22 production by type 3 innate lymphoid cells (ILC3), which in turn induces SAA from IEC and can lead to Th17 cell differentiation. Conversely, colonization of beneficial commensal bacteria induces de novo generation of Tregs and downregulates Th17 immune responses. Commensal bacteria, including most Clostridia species, produce short-chain fatty acids, i.e., butyrate, which participates in the de novo generation of T-regulatory cells by suppressing proinflammatory cytokines, by promoting RA production from DCs, and by inducing Foxp3 transcription. Among different Bacteroides fragilis strains, those expressing polysaccharide A (PSA) mediate the generation of Tregs via TLR2, while those secreting B. fragilis toxin (BFT) alter the function of IEC tight junctions. Upon disruption of barrier function, dissemination of microbial products, recognized by microbe-associated molecular patterns (MAMPs), occurs and activates the IL-23 pathway, resulting in subsequent barrier repair and stimulation of Th17 immune responses. (Adapted from Omenetti et al. 2015; [https://doi.org/10.3389/fimmu.2015.00639](https://doi.org/10.3389/fimmu.2015.00639)).**
Nevertheless, it can be predicted that some beneficial symbiont microbial species have evolved, and produce molecules that can prompt defensive intestinal immune reactions. The presently available treatments for inflammatory bowel diseases are either ineffectual in most patients or result in severe side effects. Therefore, understanding of the beneficial gut microbial species and their secreted compounds can aid in preventing or curing such diseases, and help in designing new and effective natural therapeutics against several inflammatory bowel diseases in near future.
**References:**
* Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009 May;9(5):313.
* Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Frontiers in immunology. 2015 Dec 17;6:639.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Antibiotics influence on the gut microbiome functions
Author: BugSpeaks
Published: 2018-06-27
Category: Others
Meta Title: Antibiotics influence on the gut microbiome functions
Meta Description: The current research findings have witnessed a revolution in the biological sciences and, unambiguously, in human health care. In this regard, ‘
URL: https://www.bugspeaks.com/blog/antibiotics-influence-on-the-gut-microbiome-functions
The current research findings have witnessed a revolution in the biological sciences and, unambiguously, in human health care. In this regard, ‘omics’ approaches, such as genomics, proteomics and metabolomics have helped us to unlock the paradigm of microbiome role in improving human health. A balanced maintenance of a good microbiota in our body significantly promotes our health conditions and keep away many metabolic diseases.
The microbiome usually exists in our body through an intimate symbiotic relationship, and the status of microbial flora can be an indicator of a good health. However, the microbiota is constantly being exposed to several factors that might impact on it vigorously. The microbial composition and its alterations vary from one individual to another, and this mainly depends on the microbiota stability of an individual and the nature of a disturbing factor. Under some circumstances, when the environmental or other disturbing factors are very strong, they can induce stress in our body sites, especially the gut location leading to imbalance in the taxonomic configuration of the microbiota, and their functions (a dysbiotic state). For instance, the composition of microbiome is rapidly altered when exposed to antibiotics, and may cause many possible direct or indirect health effects (Figure 1).
Antibiotics are known to kill pathogens. They are classified into natural, semisynthetic and synthetic based on their production mode and origin. For example, the fungus, Penicillium notatum producing penicillins is the base for most of the beta-lactam antibiotics. The treatment of diseases using antibiotics must be considered with a proper dosage. However, when antibiotics are consumed wrongly (not specific to the pathogen) or at high concentrations, i.e., they produce co-lateral effects in our microbiota. It has been reported that broad-spectrum antibiotics affect the richness of bacteria (up to 30%) in the gut microbial community, and cause a significant dysbiotic state. Indeed, the microbiota alterations due to antibiotics may remain for long periods, even up to a few years. Likewise, in infants, it has been evidenced that early exposure to antibiotics significantly reduces the microbial diversity of the infants’ microbiota, with weakening of Bifidobacterium and marked increases of Proteobacteria. Such effects of antibiotics on the gut microbiome have been investigated thoroughly in recent times with the availability of a variety of ‘omic’ approaches. The changes are mainly identified by 16S ribosomal RNA gene sequences analysis and shotgun sequence datasets evaluation. These researches have revealed that, in addition of altering the composition of microbiota, antibiotics upset the gene expression, biomolecules function and inclusive metabolic activities of the gut microbiota. Antibiotics swiftly modify the physiological state and functions of the gut microbiota.
The antibiotic-induced gut microbiome alter the immune and metabolic health of an individual. An immediate threat of an altered gut microbiome is the augmented vulnerability to intestinal infections, which could be because of the newly assimilated pathogens or sudden over-growth and pathogenicity by the already existing opportunistic microbes in the microbial flora. For example, antibiotic-associated diarrhea caused by the nosocomial pathogens, such as Staphylococcus aureus, Klebsiella pneumoniae, and Clostridium difficile is a common incidence. Studies have provided enough evidences to suggest that a chronic infection with C. difficile emerges with a loss of intestinal microbial diversity due to antibiotics. In a similar way, bloodstream infections in immunocompromised patients are increasing due to antibiotics treatments. Researchers have shown that bloodstream infections treated with vancomycin (an antibiotic) set a stage for the intestinal outgrowth of vancomycin-resistant Enterococcus. Studies have revealed that bacteria belonging to a limited set of phyla and genera are strongly affected by antibiotics. Fluoroquinolones, being the most widely prescribed antibiotics are reported to influence our microbiota to a greater extent with the modifications in about 32 major microbial groups. Hence, it has led to the increased resistance worldwide. The major phyla effected include Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria. In the major genera, Bifidobacterium, Escherichia, Bacteroides, Ruminococcus, Enterobacter, Faecalibacterium, Bacteroides, Lactobacillus, Eubacterium and Faecalibacterium are highly influenced by antibiotics. The effects of usages of a single antibiotic or a cocktail of antibiotics significantly disturb the microbial community. For example, the treatment with ampicillin alter only the bacteria belonging to the genera Klebsiella, Streptococcus, Bacillus, and Salmonella. Some antibiotics reduce the richness of bacteria community, which is beneficial for human health. For instance, bacteria belonging to the genus, Faecalibacterium, Bifidobacterium, and Blautia to name a few.
Few studies have investigated the influence of antibiotics on our microbes, especially gut microbiome. They suggested the role of microbial diversity and activity, expression of genes, levels of proteins or metabolites produced by microbes in their community. Antibiotics damage and/or destroy bacterial cells and therefore decreases enzymatic activities. However, in the process of antibiotic medications, antibiotic-vulnerable bacteria might be changed into resistant bacteria.
During the treatment course of antibiotics, alterations in specific enzymatic activities have been witnessed, for example the hydrolysis of dietary polysaccharides. Largely, treatments with ampicillin, cefazolin, and sulbactam are reported to alter the activity level of glycoside hydrolases and favor the rapid or disturbed absorption of carbohydrates leading to type-2 diabetes and obesity. Likewise, antibiotics treatment can cause alterations in the microbial metabolite production. In addition, antibiotics bring changes in the fluxes of genes and metabolites and the activities they mediate. A study has detected over 87% of all fecal metabolites in abundance due to streptomycin treatment. This suggests that antibiotics impacts the metabolite fluxes to a different extent. As stated by Ferrer et al. (2017), a comparative ‘omic’ investigation of microbial communities in fecal samples taken at multiple time points from an individual (with a bacterial infection) subjected to β-lactam therapy of ampicillin, sulbactam and cefazolin has revealed antibiotic-associated imbalances in long linear and branched, saturated and unsaturated fatty acids, branched chain amino acids, cholesterol derivatives, vitamins, polyols, sugars, short peptides and polyamines. Likewise, antibiotic intervention during pregnancy causes variations or copiousness of short-chain fatty acids, mostly, propionate, acetate, and butyrate, which are linked to the delivery of premature babies.
Recent years have been noticed with a rapid rise in the cases of multidrug-resistant microorganisms worldwide. This has become a great threat to the use of antibiotics for treating several microbial diseases. The resistance catastrophe is mainly attributed to the misuse and overuse of antibiotics/drugs. The effects of over-use of antibiotics are not only observed in pathogenic gut bacteria, but also in symbiotic healthy microbiota. In this regard, a preventive measures are very necessary to antibiotic-based treatment tactics that do not disturb or unbalance our inhabitant microbial flora, especially good gut bacteria having a dynamic role in health promotion.

[](/media/uploads/blog/159a.jpg)
[

](/media/uploads/blog/159a.jpg)
**Figure-1: Antibiotic effects on the gut microbiota and associated health problems. The main biological consequences of antibiotic-induced dysbioses and the potential diseases that can ensue from them are shown (only diseases with published evidence of association with antibiotic exposure are included). Involved mechanisms are shown in pink-shaded boxes.**
**(Source: Francino, 2016; [https://doi.org/10.3389/fmicb.2015.01543](https://doi.org/10.3389/fmicb.2015.01543)).**
**References:**
* Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Frontiers in microbiology. 2016 Jan 12;6:1543.
* Ferrer M, Méndez-García C, Rojo D, Barbas C, Moya A. Antibiotic use and microbiome function. Biochemical pharmacology. 2017 Jun 15;134:114-26.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The Gut Microbiome Influence the Function of Brain and Behaviors in Humans
Author: BugSpeaks
Published: 2018-06-19
Category: Microbiome and Lifestyle
Meta Title: The Gut Microbiome Influence the Function of Brain and Behaviors in Humans
Meta Description: In recent years, the role of microbiome has been increasingly acknowledged for its capability to affect the development and function of the nervous sy
URL: https://www.bugspeaks.com/blog/the-gut-microbiome-influence-the-function-of-brain-and-behaviors-in-humans
In recent years, the role of microbiome has been increasingly acknowledged for its capability to affect the development and function of the nervous system and numerous intricate host behaviors. The gut microbiome modulate the anxiety-like behavior, anxiety, social and communicative behavior, and influence the learning ability and memory. Several animal studies combined with the advanced sequencing, bioinformatics, mass spectrometry, and gnotobiotics approaches have witnessed that various societal behaviors and psychological disorders are associated with the interactions of gut-brain microbiota. However, till now, due to dearth of human studies, the relationship between gut microbiota and different behaviors is yet to be fully established. Interestingly, researchers have documented a definite role of the gut microbial composition influencing on human neurophysiology i.e., the development of brain, gene expression, and neural signaling across brain regions, such as the hippocampus, amygdala, frontal cortex, and hypothalamus.
The gut microbiome represents the collective genomes of all microbes, including bacteria, fungi, protozoa, bacteriophage, and viruses that live in our gut ecosystem. This microbial community includes both beneficial (good bacteria) and possibly harmful bacteria (bad bacteria). In the human gut, over 100 trillion microbes may exist, which is nearly 10 times the total number of cells found in the entire human body. In general, microbes harbor within the gastrointestinal tract soon after the birth and play a vital role in the human health, immunity, and various important biological functions, including the neurophysiology.
As said previously, several researchers have used rodent, chimpanzee, and other wild animal models to understand the gut-brain-microbiota interaction influencing the social and communicative behaviors, and cognitive behaviors. For example, microbiome-related modifications occur in animals due to a variety of volatile chemicals secreted by microbes, which function as olfactory signals. An animal's microbiome could influence on the communication. Likewise, odorant profiles control social isolation against attraction in insects. A high level of bacterial colonization on harvester ants increases the chances of an ant being attacked and driven out of the colony. Related effects were observed in the lower termite (Reticulitermes speratus), signifying that colonization with external microbes produces different scents indicating the entry of invaders into the colony.
In fruit fly, both larvae and adult flies preferred nourishment that was used by other larvae in comparison with new or fresh food. One more study revealed that fruit fly’s mating preference is controlled by the gut microbiota. In hyenas, the social versus lonely grown animals showed varied adjustments in specific microbial taxa. Also, there was a variation in certain volatile fatty acids produced in the social and lonely raised hyenas, which reveals a correlation between microbiome arrangement, odorant profiles, and social behaviors in mammals. Regardless of olfactory message being less prevailing in primates as compared to other mammals, evidences suggest that any deviations in the human skin microflora is linked to changes in odorant profiles. Nonetheless, the microbiome influencing on the secretion of pheromones leading to human behavior changes is yet to be understood in detail.
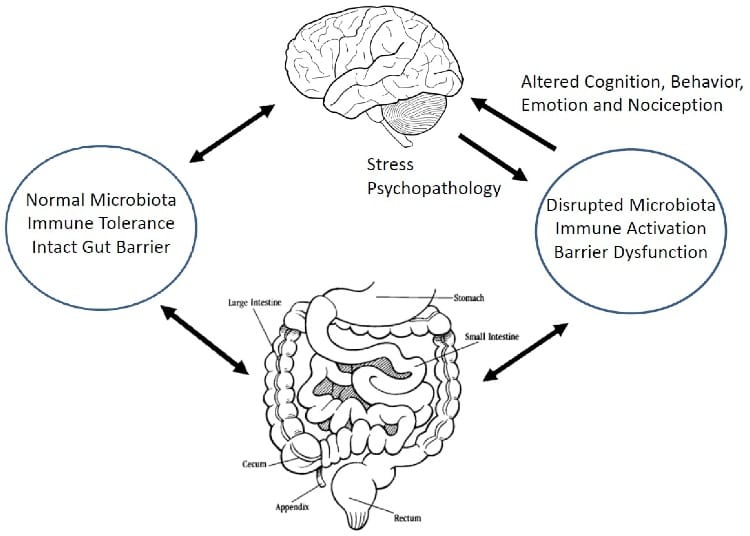
[](/media/uploads/blog/158a.jpg)
[

](/media/uploads/blog/158a.jpg)
**Figure -1: A schematic representation of the microbiota-gut-brain axis. (Source: Quigley 2018, doi:[10.3390/jcm7010006](http://dx.doi.org/10.3390/jcm7010006))**
In a social approach study, germ-free rats when raised without microbial colonization showed diminished social behavior with microbiome-complemented rats. Juvenile offsprings of antibiotic-treated rats demonstrated different microbiomes with decreased social behaviors. Similarly, another study in mice has revealed that altered gut microbiota profiles is linked to poor social behavior. When mice were subjected to initial life stress (induced neonatal stress) by splitting newborn babies from their mother for 3 hrs per day, there was an increase in the gut abnormality as noticeable from the deviation in microbiota, which was correlated to the changed brain function as part of a bidirectional feedback loop. In Chimpanzee, having repeated social interactions certainly stimulated the microbial biodiversity required to maintain a good gut health.
Studies have witnessed that probiotics positively effect on controlling many stress-related behaviors. For example, chronically stressed rats, when treated with Lactobacillus helveticus NS8 significantly enhanced exploratory behavior in the open field and reduced adrenocorticotropic and corticosterone hormone levels. Matching human trials are deficient, but in a randomized placebo-controlled study, healthy humans treated with Bifidobacterium longum R0175 and L. helveticus R0052 showed lessened anxiety-related scores. Several findings have suggested that specific probiotic treatments may modulate learning and memory behaviors in animals. Thus, these emerging studies are encouraging towards finding a solution to treat various neurodevelopmental diseases characterized by stereotyped behavior, compromised social communication, and related to several medical comorbidities, including immune dysfunction and gastrointestinal problems. However, the exact role of gut microbiota influencing the brain and behavior or how fluctuations in the brain stimulate the colonization of microbial flora in the gut is poorly understood.
Overall, these research findings present exciting promises in solving many neurological disorders of humans, including anxiety and depression. Though many neurological phenotypes have been characterized in response to microbiota depletion/alterations, gnotobiotic interventions, and other microbiome-related stimulus, prime queries concerning how changes in microbiome modulate the host behaviors remain unanswered. In this regard, future clinical studies directed towards understanding the mechanisms underlying the gut-microbiota-brain axis using novel approaches are highly necessary.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Weight loss for a better health through gut microbiota analysis
Author: BugSpeaks
Published: 2018-06-13
Category: Microbiome and Lifestyle
Meta Title: Weight loss for a better health through gut microbiota analysis
Meta Description: Overweight and obesity are defined as disproportionate body fat accumulation, and considered as a global epidemic. As per the World Health Organizatio
URL: https://www.bugspeaks.com/blog/weight-loss-for-a-better-health-through-gut-microbiota-analysis
Overweight and obesity are defined as disproportionate body fat accumulation, and considered as a global epidemic. As per the World Health Organization report, over 1.9 billion adults remained as overweight in 2014, and among them, in excess of 600 million were found to be obese. The prevailing obesity is correlated to a number of health-threatening illnesses, such as arterial hypertension, coronary heart disease, stroke, type 2 diabetes mellitus, arthritis, and asthma to name a few. Besides, this condition has significant economic effects, i.e., increase of medical expenses for its treatment or related conditions, and secondarily it causes a reduced efficiency, incapacities and early mortality in patients. Typically, obesity is due to over consumption of high calorie food in addition to a sedentary daily life. Factors, such as including genetic predisposition, increased parent age, sleep deficiency, endocrine disorders, drug iatrogenesis and epigenetic changes also contribute to the onset of obesity.
In recent times, the gut microbiome constituting the overall collection of the genetic material of microorganisms inhabiting in the gut region has been observed to have implications in triggering obesity. Interestingly, the gut bacteria constitute nearly 250-800 times more number of genes than human genes. These bacterial genes produce substances/constituents that get into the human bloodstream, and upset the body’s biochemistry. These gut bacteria are known to play a vital role in many biological processes, such as digestion, metabolism, and absorption. Thus, they involve and contribute to the development of obesity and other metabolic disorders, which is attributed to the fact that they have the ability to produce more energy from the diet, regulate and influence the fatty acid composition, encourage inflammation, and also play a part in regulating appetite via the gut–brain axis.
Various pre-clinical and clinical investigations have correlated the gut microbiome to obesity. For example, from the data of animal studies, it is evidently proved that mice which are germfree can resist obesity, however, upon introducing gut microbes into their body, the animals increased their uptake of calories from the diet and advanced towards fat deposition, and became insulin resistant. Likewise, when the gut microbiota of obese mice were colonized into normal mice, they were able to harvest energy more proficiently and accumulated body fat rapidly. The analysis of 16S rRNA gene sequenced data of the gut microbiota obtained from the distal intestine in mice indicated that a distinctive pattern occurs between obese and lean animals with respect to the most important bacterial phyla, Bacteroidetes and Firmicutes. Similarly, there exists a declined population of Bacteroidetes in the obese population. However, in the same animals, an increased number of bacterial population belonging to the phylum, Firmicutes is observed. Also, clinical findings have supported the existence of a close relationship between the gut microbiome and obesity. But, the existence of dissimilarities in the gut microbiota at the phylum level between obese and lean persons still remains as contradictory, and yet to be clarified through investigations. Hence, the gut microbiome have a critical role in causing obesity, but the causative versus substantial relationship, and the degree of its influence in humans, still remains ambiguous.
At present, obesity can be addressed through the therapeutic preferences, such as lifestyle modifications (diet, physical exercises and behavioral therapies), pharmacological treatments, and weight loss surgery in the case of severity. Lifestyle modifications may help to lose the original bodyweight up to 5–10% on a long-term basis, though there is a possibility of regaining 30-50% of the lost weight within a few years. Pharmacological treatments endorse uncertain weight loss, nevertheless lead to side effects. In severe cases, Bariatric surgery is the preferred option, which promotes a significant weight loss and reduce mortality. Yet, a large number of patients experience reduced weight-loss effects, and after the treatment, the patients may regain weight up to 75% over a period of time. Thus, there is a need to use alternative approaches for overcoming these issues and benefit patients to reduce and accomplish a healthy weight. In this regard, the manipulation of gut microbiota is a very useful approach.
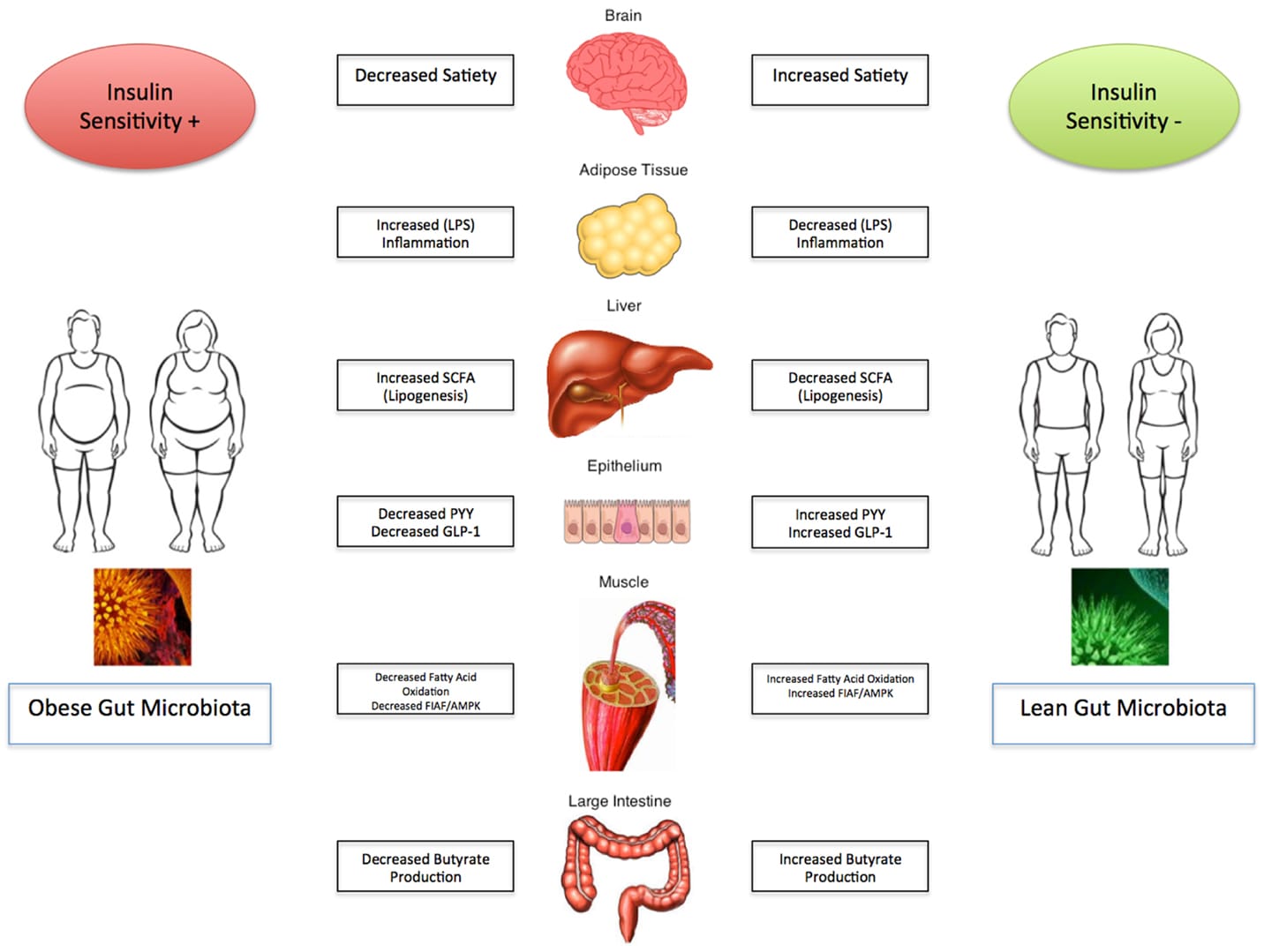
[](/media/uploads/blog/157a.jpg)
[

](/media/uploads/blog/157a.jpg)
Imbalances in the gut microbiota are central to the pathogenesis of obesity. Hence, an optimal gut microbiota balance could be helpful in fixing this problem through a precise diet choice. Including lots of fiber, healthy proteins and fats in a diet improves the gut microbiome. Good fatty acids, such as omega−3 fatty acids and monounsaturated fatty acids, i.e., extra-virgin olive oil, avocados oil or almond oil improves good gut microflora, while inflammatory omega-6 fatty acids, i.e., vegetable oils (corn, safflower, cottonseed, sunflower, and soybean oils), promote the growth of bad microbes leading to diseases and weight gain. These bad microbes secrete toxins called lipopolysacchardies that trigger inflammation, pre-diabetes (insulin resistance) and consequently, stimulate weight gain. Even chronic stress and sleep deprivation can contribute to gut microbial imbalance. Hence, practicing a healthy life schedule will favor the growth of good microbes in the gut. Overall, the percentage of good bacteria in the gut decides on how much weight a person may lose or gain, and under what conditions. The best way to improve the gut microbiota with good bacteria is to eat a healthy diet having increased fiber, good fats, avoiding unprocessed and unrefined foods, probiotic supplementation, and reducing bad bugs with medication.
Already, microbiomics has become a new frontier in medicine and providing many health solutions. Therefore, the genomic analysis of gut microbiota can precisely suggest on the intake of food, and modifications required in the daily life for an overweight or obese persons in order to lose weight, and remain fit and healthy. The clinical application of weight modifications through the gut microbiota analysis still remains unclear. Hence, additional investigations are warranted to recommend its use as a therapeutic tool in the current clinical practice of treating obese and overweight individuals.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The role of Gut Microbiome in the Wellness World
Author: BugSpeaks
Published: 2018-06-05
Category: Microbiome and Lifestyle
Meta Title: The role of Gut Microbiome in the Wellness World
Meta Description: The indigenous microbial communities are represented as microbiota, while the collection of microorganisms in the host environment where they inhabit
URL: https://www.bugspeaks.com/blog/the-role-of-gut-microbiome-in-the-wellness-world
The indigenous microbial communities are represented as microbiota, while the collection of microorganisms in the host environment where they inhabit and their genomic contents is known as microbiome. Recent advancements have transformed clinicians’ ideas about the role of microorganisms in human health and diseases. Interestingly, it has been realized that the microbiota inhabiting in our body provides a necessary ecosystem for mutual benefits, such as improving the digestion process, protection against pathogens, bioconversion of nutrients, etc. It has been reported that several trillions of microbial cells occupy different parts of the human body. Our body constitutes more bacterial cells than human cells, and they considerably affect our day-to-day lives. Therefore, the microbiome influence on human diseases and vice versa can be wide-ranging. For instance, the gut microbiota can modulate the functions of the gastrointestinal tract, produce chemicals, improve immune functions and even control the behavior. Likewise, a chronic lung disease may modify the composition of lung microbiome, and successively impact on immunity and host defense, leading to additional exacerbations of diseases. Also, bacteria, such as _Lactobacillus_, _Bacteroides_ _and_ _Bifidobacterium_ synthesize secondary bile acids, which are crucial for the lipid transport. As a beneficial bacteria, _Bifidobacterium_ generates Vitamins (B12, K, biotin, thiamine). The microbial diversity is very high in the gut region as compared to any other body parts (the skin, gums, and vaginal sites). Hence, most of the current investigations are focused on the gut microbiome and its significant role in establishing good health. The gut microbiome varies from one person to another, though they have some common illnesses like Obesity, Type 2 Diabetes or Inflammatory Bowel Disease.
A disease can be anticipated with the loss of helpful functions by the gut microbiome or by invading microbes in the gut. In other words, a disturbed gut microbiome leads to several diseases, particularly which are allied with our lifestyle aspects. Investigations have proved the connection between these microbes in our gut and many chronic disorders, such as Obesity, Diabetes, Coronary Artery Disease, Parkinson’s, Alzheimer’s, Lupus, Psoriasis, Autism, etc. Therefore, a proper nourishment of our gut microbiota can restore health conditions and make us feel happy. The consumption of healthy diet included with prebiotics and probiotics, avoiding antibiotics intake unless very obligatory, and a regular exercise may be helpful in improving our gut microbiota. In humans, the gut microbiome has co-evolved with its host since from ages and, hence, it performs a variety of crucial activities in the host, including digestion, nutrition, detoxification, body defense, improving immunity, and disease mediation. Accordingly, the human gut inhabits a wide range of microbes with high species diversity, with most of them being _Bacteroidetes_ and _Firmicutes._
The occurrence of harmful bacteria in our gut may cause a leaky gut syndrome, which happens due to the high permeability of the intestinal walls, causing leakage of undigested food particles, bacteria, and many other substances into the nearby tissues. The leaky gut syndrome is directly connected with several health problems, such as chronic fatigue, Stomach aches, Insomnia, Inflammatory Bowel Syndrome, Constipation, Diarrhea, Headaches, Depression, Cardiac problems, Pancreatic illness, etc. Our brain is directly connected to the gut microbiome, which is known to have a role in controlling or regulating our body functions. It may act autonomously (as another brain), or in combination with the brain. Hence, an unbalanced gut microbiota may trigger variations in our mood and behavior. Some of the neurological problems, such as Depression, Anxiety, Autism, and anger are correlated with health conditions of gut microbiome. A healthy microbiome can defend disease causing microbes in the body because, the beneficial microbes secrete substances or create the detrimental environment that can resist the growth of pathogens or even destroy them. For instance, volatile fatty acids, and its other byproducts produced by healthy gut microbes prevent the survival of fungi and yeast. Likewise, the pH in the digestive tract is also balanced by the microbiome so that many toxic bacteria like _Shigella, E. coli,_ _and_ _Salmonella_ _cannot survive in that condition. These bacteria are known to cause_ intestinal diseases, Diarrhea, and Food poisoning.

[](/media/uploads/blog/156a.jpg)
[

](/media/uploads/blog/156a.jpg)
Following good practices and lifestyle habits can keep our gut microbiota healthy, which in turn can impact on a healthy life. The conservation of a varied and prospering beneficial gut microbial population helps in keeping away harmful bacteria due to higher competition for the same nutrients and colonization sites. A diet, particularly rich in fibers is highly advised to maintain a healthy gut microbiota population. In addition, certain Prebiotics and Probiotic foods, such as Yogurt, Kefir, Cottage Cheese, Natto, Tempeh, etc., will certainly enhance our gut microbiota population and health. However, some of the bad lifestyle habits need to be avoided as they destroy both good microbiome and their diversity. It is suggested to avoid these bad habits such as, overuse of antibiotics, antacids, laxatives, and painkillers; drinking chlorinated water, eating sterilized foods or foods with altered fats; high intake of carbohydrates to name a few.
Molecular technologies, such as 16S ribosomal RNA sequencing, Microbiomics, Metatranscriptomics, Metaproteomics, and Metabolomics analysis have impressively improved our knowledge of the diversity, complexity, and functions of the gut microbiome within and between individuals. These high throughput technologies have delivered us with the capability to gain insights into the genes being expressed vigorously in the complex microbial communities. It has enabled us to elucidate the functional changes in directing the microbiome functions at specific environments, its interactions with the host, and functional changes involved in translating a _healthy_ microbiome in the direction of a disease-driving configuration. Thus, the gut microbiome genomic analysis can provide a clue to the role of the gut microbiota and its impact on our health.
The analysis of different genes expressed by microbes in the gut facilitates us to identify the types of metabolites that they will produce. It helps in determining the microbiome role in our body. However, just getting a data may not be useful. We have to understand about the type of genes expressed, the levels of metabolites produced and their functions, and how to improve them through a defined diet, supplementations, or modifying lifestyle activities to improve the gut microbiota of an individual. Overall, this is a personalized medicine approach to help patients to improve their gut microbiome health to overcome many diseases. It will help us to precisely decide what, how and when to take right food in order to develop wellness in a short time. Based on the microbiomics data, a patient can be accurately treated with a proper diet and lifestyle modifications. In specific, this Gut microbiome test will help us to know about the absence of beneficial bacteria and good metabolites in our gut, and the need of probiotics, carbs, proteins, and fats or nutritional endorsements to restore good health in a personalized way.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The Human Gut Microbiome and Microbiomics: Progress Towards Personalized Healthcare
Author: BugSpeaks
Published: 2018-05-28
Category: General
Meta Title: The Human Gut Microbiome and Microbiomics: Progress Towards Personalized Healthcare
Meta Description: The human body comprises 10-100 trillions of microbial cells, including archaea, bacteria, viruses, eukaryotes. The microbiome includes the combined g
URL: https://www.bugspeaks.com/blog/the-human-gut-microbiome-and-microbiomics-progress-towards-personalized-healthcare
The human body comprises 10-100 trillions of microbial cells, including archaea, bacteria, viruses, eukaryotes. The microbiome includes the combined genetic material of all microbes in a specific environment i.e., microbiota. It plays a significant role in improving human health and preventing diseases in many ways, though their mechanisms are yet to be probed in detail. The occurrence and function of microbiota differ depending on several factors, such as ages, sexes, races, diets, and change in the locations of the host. The microbiome profoundly restores normal phenotypes in the host. The past few years have investigated the microbiome in detail, and the results have tremendously reshaped our knowledge of human biology. Some of the innovative insights range from the understanding of how microbial cells intervene digestion activities, disease initiation, and progressions (e.g., inflammatory bowel disease) to unforeseen links with autism, depression, anxiety/mood disorders, Alzheimer's, Parkinson's disease, etc.
So far, studies have discovered around 10,000 microbial species (mainly bacteria) in the human body and mostly, their occurrence is predominant in the gut. Interestingly, the gut microbiota contains about 150 times more number of genes than that are observed in the whole human genome. Thus, the gut microbiome is sometimes recognized as an ‘essential organ’ because it produces products, it responds to the environment, and interacts with other systems. It is believed that the impact of these bacterial genomic DNA on our health might be more than our own genomic DNA. Likewise, it has been realized that the microbiota of gut and brain are intricately associated. There is an exchange of messengers/signals between the brain and gut microbiota. The entire body cells are influenced by the microbiome in one or the other way. In specific, it affects our mood, metabolism, libido, immune system, sensitivity, and even the clarity of your thoughts. Scientific evidences have shown that human health and diseases are dictated by what goes on in the microbiome of the gut. Diseases such as inflammatory bowel disease (IBD), obesity, diabetes, cardiovascular diseases, atherosclerosis, nonalcoholic fatty liver disease (NAFLD), alcoholic liver disease (ALD), cirrhosis, and colorectal and liver cancer have been connected to the activities of the human microbiota (Figure 1).

[](/media/uploads/blog/155a.jpg)
[

](/media/uploads/blog/155a.jpg)
**Figure 1. Human microbial symbiosis has a close relationship with diseases of different systems (Adopted from Wang et al. 2017; [https://doi.org/10.1016/J.ENG.2017.01.008](https://doi.org/10.1016/J.ENG.2017.01.008)).**
Overall, the diversity in microbiome can lead to both health and illness. Commensal microbes colonize the host soon after their birth. This microbiota community gradually develops with the host growth depending on varying ecosystems. Over time, these host-microbe interactions lead to a beneficial association, and symbiotically offer many advantages. For example, improvements in digestion, supply of essential nutrients, metabolizing indigestible compounds, and to shield against the invading pathogens. Knowing about the microbiota can help in preventing possible diseases in humans. However, their complex nature makes it difficult to study them. The standard microbial culture methods may not be sufficient enough to isolate and explore all microbes present, because some may not grow or require special growth conditions that are yet to be standardized. Later, culture independent methods for evaluating microbes was developed based on the targeted sequencing of 16S and 5S ribosomal RNA (rRNA) genes which showed differences for each species and effective in identifying organisms. Over the last decade, human microbiome researches have been transformed by using next-generation sequencing (NGS) technologies. These high-throughput sequencing allow us to study complex bacterial systems without the requirement of cloning individual genes. The targeted sequencing of 16S rRNA, 18S rRNA, 28S rRNA genes, and internal transcribed spacer (ITS) regions, and whole microbial genome of microbial communities all at once (metagenomics) is very useful in the identification of microbes and to track the functional activity of the entire community. The increasing number of such metagenomic studies of microbiomes in humans is a new hope of its potential benefits in the clinical practice.
Recent studies have witnessed the occurrence of human genetic variations as influenced by interpersonal dissimilarities in microbiomes. Thus, human genes might influence health by promoting beneficial microbiomes. The heritability study of the gut microbiota has revealed that subsets of microorganism’s abundance are genetically determined by the host. For example, a genetic change enables lactase persistence in adulthood. Interestingly, the genome-wide association studies (GWASs) of the microbiome has revealed that there is a relationship between the host genotype, consumption of milk, and gut beneficial bacteria (Bifidobacteria). It has been noticed that a single nucleotide polymorphism (SNPs) near the LCT gene on chromosome 2 and Bifidobacterium in the fecal microbiota has a close association. Bifidobacteria utilizes the lactose of milk as an energy source. This depends on the genotype of the host and diet interaction (Figure 2).

[](/media/uploads/blog/155b.jpg)
[

](/media/uploads/blog/155b.jpg)
**Figure 2: Interaction between the human lactase nonpersister genotype, Bifidobacterium abundance, and lactose in the host’s diet. Genetic variants near the LCT gene are associated with lactase persistence and in several microbiome GWASs were recently associated with Bifidobacterium relative abundance in fecal samples. The association may function according to the following scenario: (a) If an individual is a lactase persister and consumes lactose, the lactose is typically broken down into glucose and galactose in the small intestine by host lactase. (b) However, if the individual is a lactase nonpersister and consumes lactose, it travels to the large intestine, where it is fermented by lactose-utilizing bacteria, which includes Bifidobacterium. If Bifidobacteria are present, then the presence of lactose promotes their abundance. In individuals who do not consume lactose, Bifidobacterium abundance remains unaffected by their lactase persistence status (not shown). (Adopted from Goodrich et al. 2016; [https://doi.org/10.1016/j.cell.2014.06.037](https://doi.org/10.1016/j.cell.2014.06.037)).**
Likewise, GWASs has revealed that immunity related genes are influenced by the gut microbiome. Evidences suggest that genetic variations might act on digestive tract tissues to disturb the composition of the gut microbiome. Further, genetic variants in the genes, PLD1 and LINGO2 are associated with obesity. Based on the analysis of 16S transcripts, the phylogeny of microbial community has been revealed. The most active microbiota included Prevotellaceae, Lachnospiraceae, Bacteroidaceae, Ruminococcaceae, and Rickenellaceae. RNAseq analysis of bacteriophage populations in the periodontal microbiota showed the abundance of oral phages highly expressed in persons having good periodontal health. More recently, 16S rRNA gene-based approach has confirmed the predominant occurrence of Corynebacterium spp., Staphylococcus spp. and Propionibacterium spp. on healthy human skin.
Thus, knowledge on the microbiomics help to improve human health conditions. A defined diet can influence on the microbiome and restore health. Likewise, a proper use of chemicals/drugs in disease management is another factor to be considered. For example, both foods and drugs are used in treating type 2 diabetes. It is generally advised to avoid consuming simple carbohydrates to control blood sugar. Nevertheless, there exist inter-individual glycemic variations in response to diet, and some of which can be described on the basis of the individual’s microbiome. Similarly, the human microbiome plays a significant role in genetic diversity, modify disease conditions, impart immunity, provide nutrients, influences assimilation, and modulates drug interactions. Thus, intake of probiotics or beneficial bacteria (Lactobacillus, Bifidobacterium, Faecalibacterium prausnitzii, Akkermansia muciniphila, Pediococcus pentosaceus, etc.) may be useful in preventing or treating certain diseases, though most of them cannot be cultured presently. As a diagnostic tool, microbiomics is employed for detecting and measuring the levels of biomarkers i.e., cholesterol, cortisol, glucose, and creatine kinease occurring in blood which are known to indicate about diseases or infections. These novel diagnostic methods will allow clinicians to prescribe right drugs or diet to restore patient’s health.
Future microbiome investigations should involve many more new techniques to predict the functions of microbiotas, their crucial role in human development, and the mechanisms of various diseases, such as metabolic diseases, liver diseases, cancers, psychiatric diseases, etc. In this regard, many new-startups and biopharmaceutical companies, including Luecine Rich Bio Pvt. Ltd are exploring the field of microbiomics to develop novel therapeutics, diagnostic platforms and to provide clinical genomic services. A detailed data on this metagenomics might be very useful in the microbiome-based diagnosis of diseases and for the development of novel treatment strategies in the forthcoming personalized medicine era.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The microbiome role in human health and diseases
Author: BugSpeaks
Published: 2018-03-14
Category: Microbiome
Meta Title: The microbiome role in human health and diseases
Meta Description: In recent years, studies on the native microflora (microbiota) of the host environment has profoundly renewed our knowledge of human biology, health a
URL: https://www.bugspeaks.com/blog/the-microbiome-role-in-human-health-and-diseases
In recent years, studies on the native microflora (microbiota) of the host environment has profoundly renewed our knowledge of human biology, health and diseases. The term, microbiome is generally referred to the pool of genes present within a community of microbes including bacteria, fungi, archaea, protozoa, and viruses found over and inside the body. While, studies aiming to describe the occurrence and function of uncultured microbiota are frequently stated collectively as metagenomics. The life style of the host including diet habits, medical interventions, environment, and disease conditions can lead to variations in the microbiome. The structural and functional diversity of microbes allow them to survive symbiotically for a long-term in hosts. The microbiome contributes to the vital functions of the body such as metabolism, immunologic activity, energy homeostasis, gut epithelial health and neurodevelopment of the gut brain axis. They influence or mediate the process of digestion in gut, regulate immune system, protect against other pathogenic bacteria and secrete vitamins including Thiamine, Vitamins B12, Vitamin K, etc.. Overall, microbiota improves human health and helps to combat diseases. For instance, gut microbes make drugs to work better. On the other hand, they play a significant role in causing several disease including inflammatory bowel disease, autism, Parkinson's disease, type 2 diabetes, cancer, and depression. In addition, they can affect therapeutic approaches by reducing drug’s effectiveness.
The advent of next generation sequencing (NGS) technologies, metagenomics, transcriptomics, metabolomics, and analytical methods combined with the latest analytical software and the unified databases on experimental results have revolutionized the emergence of a new field, the microbiomics. It aims to identify the microbiota, genome DNA analysis, elucidate the microbiome and host interactions, and determine its role in disease pathogenicity. Basically, these approaches are used to amplify and sequence the specific microbial DNA segments followed by integrating computational analysis to identify and diversify microbes on the basis of sequence similarity and comparing with reference genomic databases of microbes. In specific, 16S rDNA (ribosome DNA) is used as the target for sequencing bacterial genome as this region is highly conserved with hypervariability and vary amongst species. In recent times, whole-genome shotgun sequencing (WGS) is the choice of analysis. This is because, WGS permits taxonomic scrutiny, investigations on the microbial gene inventories, and documents non-bacterial microorganisms that are omitted from 16S rDNA sequencing (Figure 1). Initiatives of various projects such as the Metagenomics of the Human Intestinal Tract (MetaHIT), Human Microbiome Project (HMP) have quickly enriched the microbial genomic data. This mammoth and wealthy data rapidly led to overcome computational bottlenecks and driven to develop tools such as Quantitative Insights into Microbial Ecology (QIIME) and Visualization and Analysis of Microbial Population Structures (VAMPS) which can handle big volumes of genomic data and can function in different settings ranging from supercomputers to laptops. Estimates suggest that the human body is associated with approximately 2-20 million number of microbial genes. The studies have reported the ratio of microbial cells to human cells to be 10:1. This suggest the profound impact of microbes and their genomic functions on human biology. Thus, exploration of their genomic DNA can offer exciting prospects for developing unique therapies against many chronic diseases.

[](/media/uploads/blog/148a.jpg)
[

](/media/uploads/blog/148a.jpg)
**Figure 1. Bioinformatic methods for functional metagenomics.**
(Source: Adopted from Morgan and Huttenhower (2012); [https://doi.org/10.1371/journal.pcbi.1002808](https://doi.org/10.1371/journal.pcbi.1002808))
The microbes living within us are not invaders, but helpful colonizers and play an essential role in human development, nutrition and immunity. Several autoimmune diseases including diabetes, muscular dystrophy, rheumatoid arthritis, and multiple sclerosis, and fibromyalgia are linked with dysfunctioning of the microbiome. The higher number of Lactobacillus counts in pregnancy is linked to protect the developing infant from allergic reactions. Also, studies have suggested that _Lactobacillus_\-dominated populations are protected from bacterial vaginosis. A failure in the vaginal microbiome may increase the risk of pre-term birth. Thus, the mother’s microbiome may influence her children’s health. Likewise, disease-causing microorganisms add over time, and change the gene action and metabolic functions. This may lead to irregular immune responses against body’s own constituents and tissues and results in autoimmune diseases. Overall, varied microbiome of an individual can make them susceptible to infectious diseases and lead to Crohn’s disease, irritable bowel syndrome and other chronic disorders of the intestine. Some microbial communities regulate the response of a person treated with a drug. When, a broad-range antimicrobials are taken both good and bad bacteria are killed. This might lead to the antibiotic resistance crisis. Therefore, there is a need to accurately identify pathogenic microbes so as to know their role in contributing diseases and strategize treatments which can selectively kill only pathogenic bacteria. Therefore, mapping the human microbiome is necessary to discover more microbial species and their genes that are associated with definite human health conditions. The microbiome varies depending on the population, race, geography, and diet habits. The microbiomics can help clinicians to prescribe the right drugs and diet to manage diseases effectively. The addition of probiotics is one of the nutritional supplementation to improve the microbiota of the gut. However, the application of microbiome in therapies is challenging because microbiomes are distinctive to every single person and vary over time. Thus, it complicates the use of the microbiome for both diagnosis and to design a therapy. At present, research studies are aimed to investigate on the following aspects:
* Nutrition in diet affecting the microbiome.
* The microbiome affecting immune response and contributing to diseases.
* Antibiotics and the microbiome interactions to overcome drug-resistance.
* The microbial response to various drugs.
* Altering the microbiome to improve health conditions.
The microbiome is very diverse, and complex and many fundamental questions remain unanswered. Improved computational approaches for the metagenome assembly/analysis are the urgent need in this regard. Moreover, their relations to metabolism, epigenetics, host genetics, and immunology can facilitate to unravel their complex relationships with human health and diseases. This can be better exploited for curing several chronic diseases. Some of the ethical concerns of the microbiomic research include; data being obtained from a typical sample of the population, informing about the consent to samples collection, data sharing, protecting privacy, invasiveness of taking the samples, etc. In future, investigations should aim to explain the mechanisms of interactions between the microbiome and host, decipher the microbiome growth during host growth and development, its impact on health conditions, elucidate the role of its pathogenicity, and measure the feasibility of diagnostic examinations, and target the therapies against human diseases.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Let Food be thy Medicine
Author: BugSpeaks
Published: 2018-01-23
Category: Nutrition
Meta Title: Let Food be thy Medicine
Meta Description: About the Speaker:
Dr. Sripriya Venkiteswaran, is a nutritionist by profession, and an avid follower of all things that are nutritious for the past
URL: https://www.bugspeaks.com/blog/let-food-be-thy-medicine
**About the Speaker:**
Dr. Sripriya Venkiteswaran, is a nutritionist by profession, and an avid follower of all things that are nutritious for the past two decades. She has a bachelor’s degree in Nutrition, a PG Diploma in Clinical Nutrition and Dietetics, followed by a PhD in Nutritional Sciences.
Her interest for the molecular aspects of nutrition were ignited during her PhD from Rutgers University, which has turned in to her core strength and passion. In this TEDx\_IBA\_Bangalore talk she shares her views and knowledge about nutritional facts and how food itself can heal as well as shape the body.
**Highlights**
* How food, in extension nutrition, affects your body from within (at genome level)
* How most regulatory aspects or dietary recommendations have been focused around "the general" or concentrated on "the average human being".
* Why this approach has largely been unsuccessful
* Later, she proposes that, since everyone is genetically unique, the way we respond to various foods would also be unique, which in extension have largely been the root cause why "one size fits them all" approach does not work.
* She explains the two-way effect of gene-to-nutrition and nutrition-to-gene on our body, providing some compelling examples of Agouti and MTHFR genes.
* With expanding prospects of genomic profiling, she feels excited about the future, but she also urges both scientific community to gather comprehensive data before customizing the nutritional recommendations.
* She also touches upon several other topics pertinent like patient persuasion, status of nutritionists within the medical community and even reviving our (Indian) traditional nutritional practices advocated with genomic data.
* She concludes reiterating the point that 'your genotype is also as important as your appetite".
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Are we humans or are we bacteria
Author: BugSpeaks
Published: 2017-03-15
Category: Microbiome
Meta Title: Are we humans or are we bacteria
Meta Description: Josh Neufeld is an Associate Professor, University of Waterloo in this TED video explains the abundance of microbes in our body and its function.
URL: https://www.bugspeaks.com/blog/are-we-humans-or-are-we-bacteria
Josh Neufeld is an Associate Professor, University of Waterloo in this TED video explains the abundance of microbes in our body and its function.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Gut microbiome and inflammation
Author: BugSpeaks
Published: 2017-03-14
Category: Microbiome and Disease
Meta Title: Gut microbiome and inflammation
Meta Description: In this short video, Prof. Adiel Tel-Oren explains the inflamed gut and how to eliminate risks of intestinal diseases.
URL: https://www.bugspeaks.com/blog/gut-microbiome-and-inflammation
In this short video, Prof. Adiel Tel-Oren explains the inflamed gut and how to eliminate risks of intestinal diseases.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## How nutrition shapes gut microbiota
Author: BugSpeaks
Published: 2017-03-13
Category: Nutrition
Meta Title: How nutrition shapes gut microbiota
Meta Description: How Nutrition Can Shape Gut Microbiota and its Implications in the Autoimmunity Epidemics is explained here by Alesso Fasano, MD Massachusetts
Genera
URL: https://www.bugspeaks.com/blog/how-nutrition-shapes-gut-microbiota
How Nutrition Can Shape Gut Microbiota and its Implications in the Autoimmunity Epidemics is explained here by Alesso Fasano, MD Massachusetts
General Hospital for Children (MGHfC)
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The brain in your gut
Author: BugSpeaks
Published: 2017-03-10
Category: Microbiome and Lifestyle
Meta Title: The brain in your gut
Meta Description: Did you know you have functioning neurons in your intestines -- about a hundred millions of them? Food scientist Heribert Watzke tells us about the &q
URL: https://www.bugspeaks.com/blog/the-brain-in-your-gut
Did you know you have functioning neurons in your intestines -- about a hundred millions of them? Food scientist Heribert Watzke tells us about the "hidden brain" in our gut and the surprising things it makes us feel in this TED talk.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## All About Bacteria
Author: BugSpeaks
Published: 2017-03-09
Category: Microbiome
Meta Title: All About Bacteria
Meta Description: Ravi Mantha, in this TED video speaks about health disorders that occur to human being on the theme "All about bacteria" and human health sy
URL: https://www.bugspeaks.com/blog/all-about-bacteria
Ravi Mantha, in this TED video speaks about health disorders that occur to human being on the theme "All about bacteria" and human health system.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Microbiome and the Brain Interactions
Author: BugSpeaks
Published: 2017-03-08
Category: Microbiome and Lifestyle
Meta Title: Microbiome and the Brain Interactions
Meta Description: Dr. Emeran Mayer, David Geffen School of Medicine at UCLA in this video talks about the fascinating world of microbes and its interaction with brain.
URL: https://www.bugspeaks.com/blog/microbiome-and-the-brain-interactions
Dr. Emeran Mayer, David Geffen School of Medicine at UCLA in this video talks about the fascinating world of microbes and its interaction with brain. The neurobiological aspects of the gut-brain axis are very well explained in this video.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## What happens in Vagus, does not stay in Vagus!!
Author: BugSpeaks
Published: 2017-03-07
Category: Microbiome and Lifestyle
Meta Title: What happens in Vagus, does not stay in Vagus!!
Meta Description: So, it’s been two months past the new year. We all are coming back to our normal selves, we are eating (binging) whatever we want to, not so kee
URL: https://www.bugspeaks.com/blog/what-happens-in-vagus-does-not-stay-in-vagus
So, it’s been two months past the new year. We all are coming back to our normal selves, we are eating (binging) whatever we want to, not so keen on working out or even waking up early...for that matter.
Now that we are enlightened about new year diets, workout regimes & resolutions, and have understood these to be just myths, we can actually focus on doing what we do best
"**sit and ponder about everything**"
Everything, from menial questions about career, marriage, relationships ... to ... more serious questions like - should I cook today or order pizza?... then to ... potentially life changing questions like... Why am I fat ?!!
We always talk about stuff that we eat going directly to our hips (especially for us girls, right?!)
Here is an interesting observation:
What if, the food we eat directly goes to (or affects) our head?
hmmm... Now that's a thing to ponder upon,
I mean how's that even possible?
Well, you might have heard or even felt that, when you are in stress you eat more, or when you are tensed you have gastric problems, or when you are hungry for long duration you get a headache. So, you know these things are somehow connected, and mostly seem to be related to the food that you eat.
But, in fact, this relationship, between your behavior and your food (more importantly the other way around), is surviving (read hanging in there) because of the tiny tenants in your body.
_**And this is where the story begins...**_
When you are in your mother's womb there are no microorganisms in your body, and you are virtually sterile, inside out!!
As a new-born, you are exposed, for the first time, to a wide array of microbes from a variety of sources, including maternal bacteria. The process of birth exposes you to the first microbes from your mother's vaginal tract or stomach, depending on the mode of birth. (fascinating? or you feeling eeeww?)
Later, your mother's milk gives you the major share of microbes, that actually help you build your immunity. In fact, [a recent study](https://www.ncbi.nlm.nih.gov/pubmed/23401408) talks about how infants born by cesarean section are at increased risk of asthma, obesity and Type 1 diabetes, as compared to babies born through normal labor and exposed to the maternal vaginal microbes.
As and when you start eating different kinds of foods, drinking beverages, use a specific kind of bathing soap etc., the microbiome (total microbes on and) in your body gets enriched. More specifically, your gut microbiota gets developed with every exposure to different environments. In fact, what you eat, drink, smoke, or gets exposed to, all defines the microbiota of your gut.

[](/media/uploads/blog/100a.jpg)
[

](/media/uploads/blog/100a.jpg)
I still remember the day, when I had my first beer... and my gut microbes were like... bring it on girl!!
Another interesting fact is that, since diet and food habits are largely an extension of your culture, your gut microbiome differs from American's or Chinese, given that we all are culturally world apart, and so is our lifestyle and dietary habits.
_**The other side of the story…**_
On one end, the food you eat and drink affects the composition of your gut microbiota. However, over time, the enriched and developed gut microbiota influence the digestion of specific food you eat, indirectly influencing your diet. So, lot of these gut bacteria influence and more importantly help you digest food in an efficient way.
Another interesting fact is that, whenever these good bacteria goes low in number, it gives way for over growth of bad microbes like Clostridium, E. coli etc., that causes diseases like diarrhea, colitis, etc.
And why would these good bacteria go low in number?
Well... it can be due to any reason from imbalanced or untimely diet to exposure to antibiotics or smoking. [One study found](https://www.ncbi.nlm.nih.gov/pubmed/3846592) that after a single treatment of intravenous antibiotics, fecal bacteria demonstrated a significant change in the variety and abundance of bacterial strains.
Overall, the food that we eat influence the gut microbiota and the microbiota influence the what we eat - requiring a fine balance between the two.
Okay, now we are drifting
_**Let's come back to the point where food and head are connected**_
Studies, involving college kids (the junk eating generation), eating burgers and fries, guzzling cokes and sodas, observed that these students felt lethargic, largely unwell, depressed, with general fatigue, disturbed / unfocused mind, just after a few weeks of continuous ingestion of the junk diet. Testing their gut revealed that, and I quote “gut microbes (the microbiome) had been devastated”, with potential disease causing firmicutes replacing good Bacteroidetes, as the dominant type, while the friendly Bifidobacteria, that suppress various kinds of inflammation, halved in its abundance being the clearest marker of an unhealthy gut.
The question is why? and How does what you eat .... affecting your gut microbes... affect your state of mind?
The human gut microbiome is so dynamic and complex, that it consists of approximately 1 kg of bacteria in an average adult, and is approximately the weight of the human brain. This microbiota produce a myriad of neuroactive compounds, regulating everything from your behavior to cognitive function, social interaction to stress management. It regulates these brain functions by steering the "Vagus Nerve".
This thing called 'Vagus nerve' (Not "Vegas and Casinos") within your nervous system, is a part of cranial nerves unit, that forms an extensive network, linking your gut and brain. Creatively enough, termed as [the gut brain axis](https://fivethirtyeight.com/features/gut-week-gut-brain-axis-can-fixing-my-stomach-fix-me/), it is the sole reason for all the food and head related things. This system, largely involving the Vagus nerve, controls the body’s unconscious actions, such as digestion, excretion, and sexual arousal.
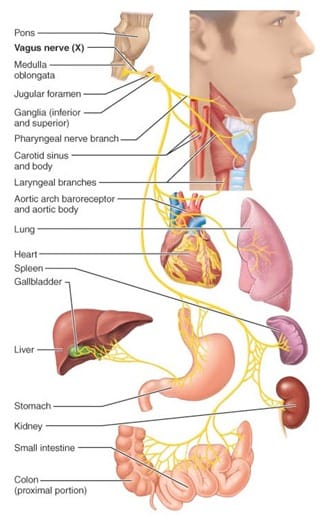
[](/media/uploads/blog/100b.jpg)
[

](/media/uploads/blog/100b.jpg)
In the absence of certain (good) microbes, due to imbalanced diet (read junk eating), the neuroactive compounds, that regulate the above said unconscious actions, are profoundly altered. It turns out, what you eat changes the gut ecosystem, in turn resulting in altered vagal activity.
_**But, what happens in Vagus does not stay in Vagus!!**_
The altered vagal activity leads to a lot of intermittent chain reactions, including decreased pancreatic enzyme secretion, poor gallbladder function, overall disturbed gut function. This suppresses the intestinal immune system, decreases intestinal blood flow, increases intestinal permeability, eventually leading to leaky gut, causing digestive problems, inflammation and infection, further aggravating the issues.
The cytokines produced during this imbalance, cross the blood-brain barrier, leading to decreased activity in brain, decreased activation of the vagal motor nuclei, inflammation and decrease in nerve conductance. The effects being anything from mild headaches to aberrant stress to long term depression. A causal association, between gut microbes and cognitive decline and disorders of cognitive function such as Alzheimer's disease and multi-infarct dementia (high prevalence after the age of 65 years), is already been studied.
Wait a minute, before, you thought, when you are in stress you eat more, or when you are tensed you have gastric problems, or when you are hungry for long duration you get a headache. Now you understand that these things are connected, but, possibly in the opposite order, where the food you eat cause the stress, gastric issues and headache.

[](/media/uploads/blog/100c.jpg)
[

](/media/uploads/blog/100c.jpg)
So, coming back the full circle, to where the story began...
the gut microbes (or the whole microbiome) play a very important role in your growth, wellbeing and longevity, from your birth to senility. In fact, it has been established that post-birth, the gut microbial colonization occurs in parallel with cognitive development. There is [increasing evidence](https://www.ncbi.nlm.nih.gov/pubmed/20966022) to support the view that the evolving cognitive activity is critically dependent on the microbiota and its metabolic activity.

[](/media/uploads/blog/100d.jpg)
[

](/media/uploads/blog/100d.jpg)
_**Now that’s a lot of pondering!!**_
(as I said, that’s what we do best, right?)
So, the next time you think about a diet plan (especially the new year ones), or any resolution for that matter, listen to your gut and gradually change and adapt, without hitting the Vagus.
Or else, come and read this blog, again, next year!!
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## How changing a body's microbes leads to illness
Author: BugSpeaks
Published: 2017-03-06
Category: General
Meta Title: How changing a body's microbes leads to illness
Meta Description: Claire M. Fraser, PhD, in this TED talk explains how microbes affect our lives. We are more obsessed with cleanliness than ever: we use antibacterial
URL: https://www.bugspeaks.com/blog/how-changing-a-body-s-microbes-leads-to-illness
Claire M. Fraser, PhD, in this TED talk explains how microbes affect our lives. We are more obsessed with cleanliness than ever: we use antibacterial cleansers, we keep our children away from dirt, and we give out antibiotics with little regard for long-term effects. But in the process, we are altering our microbiota, the microbes that live on and inside us. And it's causing us to be more sick.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Gut Microbiome - Its impact on our lives
Author: BugSpeaks
Published: 2017-03-03
Category: General
Meta Title: Gut Microbiome - Its impact on our lives
Meta Description: The human intestinal tract is home to a diverse and complex microbial community that is increasingly being recognized for its importance in overall hu
URL: https://www.bugspeaks.com/blog/gut-microbiome-its-impact-on-our-lives
The human intestinal tract is home to a diverse and complex microbial community that is increasingly being recognized for its importance in overall human health. This community is commonly referred to as a hidden metabolic 'organ' due to its pivotal impact on a vast array of functions including metabolism, physiology, nutrition and immunity. Thus, alterations of the microbiome can significantly impact health and thus contribute to multiple chronic disease states. In this presentation, Dr. Robert Rountree in this video discusses the role of the microbiome in health and disease - including practical ways to restore and maintain a healthy microbiome.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Psychology and Gut Bacteria
Author: BugSpeaks
Published: 2017-03-02
Category: Microbiome and Lifestyle
Meta Title: Psychology and Gut Bacteria
Meta Description: Marc David, founder of the Institute for the Psychology of Eating, explains in this fascinating new video, where he explains about the psychology and
URL: https://www.bugspeaks.com/blog/psychology-and-gut-bacteria
Marc David, founder of the Institute for the Psychology of Eating, explains in this fascinating new video, where he explains about the psychology and how we relate to “gut bacteria.” And that psychology will powerfully inform our health and our well-being. To that end, it’s a great idea to examine the psychic forces that drive us in relation to anything that’s important in our lives.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Diet, Digestion and Microbiome
Author: BugSpeaks
Published: 2017-02-28
Category: General
Meta Title: Diet, Digestion and Microbiome
Meta Description: Professor Kevin Whelan talks about diet and the microbiome at Core's Exploring the Science of Digestion event at the Kia Oval, London.
URL: https://www.bugspeaks.com/blog/diet-digestion-and-microbiome
Professor Kevin Whelan talks about diet and the microbiome at Core's Exploring the Science of Digestion event at the Kia Oval, London.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Microbiome and Autoimmunity
Author: BugSpeaks
Published: 2017-02-27
Category: Microbiome and Disease
Meta Title: Microbiome and Autoimmunity
Meta Description: At the 2012 International Congress on Autoimmunity, in Granada, Spain, Prof Trevor Marshall delivered this plenary lecture -- explaining how the Human
URL: https://www.bugspeaks.com/blog/microbiome-and-autoimmunity
At the 2012 International Congress on Autoimmunity, in Granada, Spain, Prof Trevor Marshall delivered this plenary lecture -- explaining how the Human Microbiome causes Human Immune dysfunction, including many autoimmune diseases.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The Microbiome Diet
Author: BugSpeaks
Published: 2017-02-24
Category: Microbiome
Meta Title: The Microbiome Diet
Meta Description: In The Microbiome Diet book, Dr. Kellman gives some delicious gut-health promoting foods; populating friendly bacteria for optimal health, good bacter
URL: https://www.bugspeaks.com/blog/the-microbiome-diet
In The Microbiome Diet book, Dr. Kellman gives some delicious gut-health promoting foods; populating friendly bacteria for optimal health, good bacteria foods for a healthy gut, the best way to restore your gut flora, how to recover gut health, how probiotics heal leaky gut, how do you heal a leaky gut, best micro biome diet, why your body needs probiotics, best probiotic supplement in the US, health benefits of biotic balance, why you should take biotic balance, health benefits of probiotics, health benefits of probiotics are highlighted in this video.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Microbes, Wellness and Mealtime
Author: BugSpeaks
Published: 2017-02-23
Category: General
Meta Title: Microbes, Wellness and Mealtime
Meta Description: Much of our population struggles with issues stemming from brain imbalances. New research is finding a strong connection between our gut bacteria and
URL: https://www.bugspeaks.com/blog/microbes-wellness-and-mealtime
Much of our population struggles with issues stemming from brain imbalances. New research is finding a strong connection between our gut bacteria and our mental health. In this video, Lisa Kilgour, R.H.N. is a Registered Holistic Nutritionist, discusses how food impacts our gut, and has the power to either exasperate imbalances like depression, or boost our overall wellbeing.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Mind altering microbes
Author: BugSpeaks
Published: 2017-02-22
Category: Microbiome and Lifestyle
Meta Title: Mind altering microbes
Meta Description: laine Hsiao. a postdoctoral fellow in chemistry and biology at Caltech, in this TEDx talks about various mechanisms by which microbes modulate host pr
URL: https://www.bugspeaks.com/blog/mind-altering-microbes
laine Hsiao. a postdoctoral fellow in chemistry and biology at Caltech, in this TEDx talks about various mechanisms by which microbes modulate host production of neuroactive molecules and aims to better understand how the human microbiota influences health and disease. Here she throws light on Neuroimmune mechanisms underlying the pathogenesis of neurodevelopmental disorders and uncovered a role for the commensal microbiota in regulating autism-related behaviours’, metabolism, and intestinal physiology.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Human Microbiome as a community
Author: BugSpeaks
Published: 2017-02-21
Category: Microbiome
Meta Title: Human Microbiome as a community
Meta Description: The recent explosion of research into our body's microbial universe will change the treatment of metabolic, immune and psycho-emotional disorders.
URL: https://www.bugspeaks.com/blog/human-microbiome-as-a-community
The recent explosion of research into our body's microbial universe will change the treatment of metabolic, immune and psycho-emotional disorders. What’s more, it will cast a whole new perspective on our metaphors, cultural assumptions, and the very identity of “self”, as described in this talk by cultural anthropologist Miriam Lueck Avery.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Our Microbiome and Health
Author: BugSpeaks
Published: 2017-02-20
Category: Microbiome and Lifestyle
Meta Title: Our Microbiome and Health
Meta Description: Did you know that you have up to ten times as many microbial cells on your body as you have human cells? What are these tiny microbes doing and how di
URL: https://www.bugspeaks.com/blog/our-microbiome-and-health
Did you know that you have up to ten times as many microbial cells on your body as you have human cells? What are these tiny microbes doing and how did they find their way to you? Rob Knight, PhD joins our host David Granet, MD to discuss how these cells that make up our microbiome can impact everything from mood, weight, sleep patterns, allergies and more.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The gut feeling
Author: BugSpeaks
Published: 2017-02-17
Category: Microbiome
Meta Title: The gut feeling
Meta Description: This video explains how people are venturing in to microbiome. Advances in DNA sequencing and big data allow us to analyse bacteria like never before.
URL: https://www.bugspeaks.com/blog/the-gut-feeling
This video explains how people are venturing in to microbiome. Advances in DNA sequencing and big data allow us to analyse bacteria like never before. Scientists and entrepreneurs have entered a race to develop tools and datasets that uncover the role microbes play in human health.
Bacteria are no longer the enemy. Will bacteria enable personalized medicine?
Yes, there are many companies that focus on the microbiome that could transform multi-billion dollar concerns like weight loss, probiotics, cosmetics, dental health, and even chronic diseases like diabetes. Will microbiome analysis change modern medicine? How are entrepreneurs involved? Is the money in sequencing, analytics, or drug development?
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The bugs inside your gut
Author: BugSpeaks
Published: 2017-02-16
Category: General
Meta Title: The bugs inside your gut
Meta Description: Join Warren Peters in his TEDx talks about the journey into understanding your gut microbiome and the new discoveries changing the way we understand d
URL: https://www.bugspeaks.com/blog/the-bugs-inside-your-gut
Join Warren Peters in his TEDx talks about the journey into understanding your gut microbiome and the new discoveries changing the way we understand diseases like diabetes, obesity, Alzheimer's disease, autism, and our everyday health and wellness.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The Microbiome
Author: BugSpeaks
Published: 2017-02-07
Category: General
Meta Title: The Microbiome
Meta Description: Dr. Daniel Beiting, Research Assistant Professor at UPenn in the School of Veterinary Medicine in tis TED talk explains about the microbiome in g
URL: https://www.bugspeaks.com/blog/the-microbiome
Dr. Daniel Beiting, Research Assistant Professor at UPenn in the School of Veterinary Medicine in tis TED talk explains about the microbiome in general.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## The gut flora
Author: BugSpeaks
Published: 2017-02-06
Category: Microbiome and Disease
Meta Title: The gut flora
Meta Description: Jeroen Raes, researcher on human microbiome, Flanders Institute of Biology explains his discovery in this TED talk, the major breakthrough not just in
URL: https://www.bugspeaks.com/blog/the-gut-flora
Jeroen Raes, researcher on human microbiome, Flanders Institute of Biology explains his discovery in this TED talk, the major breakthrough not just in gastro-intestinal medicine, but in our fundamental knowledge of the human biology. It turns out that there are only three different types of gut bacteria and, just like blood groups, the three types are totally independent of race, sex, age or diet. Such a baffling finding leads to more research of course and Raes is currently testing his idea on a larger group. The implications for Crohn's Disease or obesity could be dramatic.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Microbiome, the future of medicine?!
Author: BugSpeaks
Published: 2017-02-03
Category: General
Meta Title: Microbiome, the future of medicine?!
Meta Description: We've all heard about the microbiome, but what is it? Why should we care? And, most importantly, what should we do about it? Integrative gastroent
URL: https://www.bugspeaks.com/blog/microbiome-the-future-of-medicine
We've all heard about the microbiome, but what is it? Why should we care? And, most importantly, what should we do about it? Integrative gastroenterologist Dr. Robynne Chutkan, in this video breaks down what you need to know about the microbiome, the vast collection of microbes that live in and on your body, and how you can optimize your gut health to live a longer, better life.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## How our microbes make us who we are
Author: BugSpeaks
Published: 2017-02-02
Category: Microbiome
Meta Title: How our microbes make us who we are
Meta Description: Rob Knight, a pioneer in studying human microbes, the community of tiny single-cell organisms living inside our bodies that have a huge and largely un
URL: https://www.bugspeaks.com/blog/how-our-microbes-make-us-who-we-are
Rob Knight, a pioneer in studying human microbes, the community of tiny single-cell organisms living inside our bodies that have a huge and largely unexplored role in our health. In this video, he explains why “The three pounds of microbes that you carry around with you might be more important than every single gene you carry around in your genome,”.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Human Microbiome - The Invisible Universe
Author: BugSpeaks
Published: 2017-02-01
Category: General
Meta Title: Human Microbiome - The Invisible Universe
Meta Description: Human body consists of 10 times more cells from microorganisms like bacteria and fungi than there are human cells. These tiny compatriots are invisibl
URL: https://www.bugspeaks.com/blog/human-microbiome-the-invisible-universe
Human body consists of 10 times more cells from microorganisms like bacteria and fungi than there are human cells. These tiny compatriots are invisible to the naked eye but their contribution towards human health and immunity is enormous. Ben Arthur, artist, in this video gives us a guided tour of the rich universe of the human microbiome.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Bridging Traditional Dietary and Current Nutritional Practices
Author: BugSpeaks
Published: 2016-12-22
Category: General
Meta Title: Bridging Traditional Dietary and Current Nutritional Practices
Meta Description: Kefir is a friendly probiotic drink with slightly fizzy and tangy taste, a FANTASTIC fermented milk beverage originating from Eastern Europe and South
URL: https://www.bugspeaks.com/blog/bridging-traditional-dietary-and-current-nutritional-practices
Kefir is a friendly probiotic drink with slightly fizzy and tangy taste, a FANTASTIC fermented milk beverage originating from Eastern Europe and Southwest Asia. It looks like and tastes like thin yogurt, but far superior than others, as a probiotic. It is incredibly beneficial for digestion and gut health maintenance when consumed regularly. It is loaded with valuable vitamins and minerals and contains easily digestible complete proteins. People with lactose intolerance can eat kefir without problems, adding to its list of benefits.
[](/media/uploads/blog/59a.jpg)
[

](/media/uploads/blog/59a.jpg)
Kefir can be prepared from any type of milk, cow, goat or sheep, coconut, rice or soy. It is made by adding kefir “grains” to milk. These are not grains in the conventional sense, but cultures of yeast and lactic acid bacteria. Over a period of 24 hours’ time, the microorganisms in the kefir grains multiply and ferment the sugars in the milk, turning it into kefir.

Even though Kefir has been widely recognized as a health drink with benefits, recent development in the field of probiotics, have raised enormous interest in Kefir, with researchers reporting a wide spectrum of progressive elements present in it benefits the person who is consuming it, as it is known for its anticarcinogenic, anti-inflammatory, and antipathogenic effects.
Unfortunately, misuse of the term probiotic has also become a common place and a major issue, with many products exploiting the term without meeting the requisite criteria. Consequently, there is a certain level of apprehension about these effects of Kefir, bringing it under the scrutiny. Can Kefir really show these effects in human, as most of the studies are done on mice and cell lines? If so then, what is the mechanism? What is the uniqueness of Kefir in terms of its bacterial composition?
Initially, some nutritional benchmarking has clearly identified the presence of beneficial components within Kefir (see picture below). “[Antimicrobial and healing activity of kefir and kefiran extract](https://www.ncbi.nlm.nih.gov/pubmed/15848295)” has also be studied early on, demonstrating highest activity against Streptococcus _pyogenes._ Further, Lactobacilli have been recognized as a major component of Kefir, which have GRAS (Generally Regarded As Safe) status. Of these, Lactobacillus _kefiri_ has been identified as the key player in Kefir that has already assessed many functional properties, [safety characterization and antimicrobial properties](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4052788/). However, parameters regarding safety of Kefir was imperative, before calling them probiotics.

[](/media/uploads/blog/59b.jpg)
[

](/media/uploads/blog/59b.jpg)
Paul Cotter, of Teagasc Food Research Centre in Cork, Ireland, approached the issue to answer such questions associated with kefir, [employing tools of high-throughput sequencing to analyse the microbial composition of Kefir](http://msystems.asm.org/content/1/5/e00052-16). His team of researchers performed Shotgun metagenomic sequencing of the microbial population of Kefir. Along with identifying the composition, they also determined the systematic progression of the microbial communities during fermentation. They observed that, as the fermentation progressed, the bacterial diversity diminished, and species that dominate the population at the beginning are replaced by others, just hours after. For instance, _Lactobacillus_ _kefiranofaciens_ was the dominant bacterial species recorded to be in kefir during early stages of fermentations, but _Leuconostoc_ _mesenteroides_ became more prevalent during later stages. They could further establish the link between individual species present in kefir, flavour generating compounds, several genes and associated pathways. They identified genes that are responsible for some of the intestinal benefits seen in kefir-drinkers. Through metabolomic approach they could map the acidic taste of kefir to the presence of Acetobacter _pasteurianus_ and cheesy flavours to Lactobacillus _kefiranofaciens_. Additionally, they were able to change the flavour and taste of kefir by altering the ratio of these identified microbes.
Metagenomics, again, have proven to be a new way to look at bacteria, not only within hosts, but within commodities that have traditionally proven to possess health benefits. This whole new approach of analysis could in fact provide scientific insights and widen the consumer audience by imparting necessary knowledge to bridge the gap between traditional dietary & current nutritional practices.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Are you dating a meat eater?!
Author: BugSpeaks
Published: 2016-12-19
Category: Diet and Supplements
Meta Title: Are you dating a meat eater?!
Meta Description: He is tall, handsome, tattooed, white collar college grad, financially secure, overall “fits the bill” (The bill?!!) and the perfect guy t
URL: https://www.bugspeaks.com/blog/are-you-dating-a-meat-eater
He is tall, handsome, tattooed, white collar college grad, financially secure, overall “fits the bill” ([The bill?!!](https://www.youtube.com/watch?v=Dw6VE8jjP-0)) and the perfect guy to spend the rest of my life with.
Sounds like a fairytale, right?
Ohhh, wait a minute, what does fairytales usually have? Monsters and witches who always twist and turn the story to eleven.
An ace in the hole for this fairytale, HE EATS MEAT!! And, I don’t.
I mean, I don’t now, but I used to. But, have been a vegan for a while.
So now, being with him means, I would return to meat eating?? Psychology says majority of the converted vegans and vegetarians do return to meat eating. But, I have achieved this with great difficulty and have become a no-meat eater (obviously, I will make one of my kind) and I don’t want to call it a quit now.
So, what should I do?
May be, just maybe, I make him go vegan, and I do have ample reasons to persuade him to go vegan, then me returning to meat eating.
In retrospect, my reasons for not eating meat began with general health, concern towards animal welfare, spanning across to high carbon footprint in meat and global wellbeing. Thus, beginning my vegan diet saga, saving animals and the world I live in, and by eating more of grains, legumes, beans, and of course, BROCOLLI !!
[](/media/uploads/blog/58a.jpg)
[

](/media/uploads/blog/58a.jpg)
Initially I wasn’t happy, had constant hunger and felt it was a daily chore to deal with. I was tired of being hungry and was always seen with low energy. However, with great difficulty I adhered to vegan diet and the rewards were worth the pain. I started feeling lighter, more energetic and improved my overall wellbeing.
So, I would start with this background, but I know this would not convince him (remember, he is intelligent too). So, I began imagining the arguments he would put in and try to come up with valid reasons that would convince him.
**“The ancient argument”**
That humans are naturally omnivorous and we are evolutionarily designed to eat both animal meat and plants.
The relationship between the microbiome, human health and diseaseFirst of all, enough evidence to prove this point isn’t available. Further, talking of evolution, we “EVOLVED” from meat eating wanderers to lake-side living ‘agriCULTURE’ driven social animals, a long time ago.
“**Meat tastes far better than plants”**
Well, not true. Don’t believe me? It actually assumes the taste of flavors and additives we put in, and, even ‘The Matrix’ has acknowledged this. [Chicken tastes like everything](https://www.youtube.com/watch?v=2oEnJfZ9joY)!! (Yes, I have seen ‘the matrix trilogy’ and have UNDERSTOOD it!!)
Moreover, there are lots of vegan and vegetarian dishes that are tastier, and I have quite a few recipes from my own R & D (I mean, my kitchen), which makes vegan diet deliciously healthy and gut friendly too.
**The iron in meat is easier for the body to process than the iron in plants. Meat has higher amount of protein compared to plants**
Oh, now we are getting technical, aren’t we!!
Sure, meat might have these advantages, but all this comes at a cost of unwanted fat. Meat has far more fat than plants and fat isn’t good neither for your adipose nor for your gut. In fact, some of the foods like tofu has higher amount of protein, vitamins, iron than meat and in addition contains 9 essential amino acids, as well as, some may even have cancer-protecting qualities. How about that for technical, eh ?!
“**Change in sudden diet might cause health issues”**
Yes initially, there could be some specific health-related symptoms, while moving from meat to no-meat diet, but then your body and your gut gets used to the new diet. Trust me, it will work charms for you and I am sure the bacteria in your gut would be happier.
**What’s with the gut and the bacteria?**
We have trillions of bacteria in our gut, and these bacteria (called the microbiome) has been known to determine everything from our digestion to mood, immunity to diseases. The vegans/vegetarians have been shown to contain lots of good bacteria in their guts, would fall less sick because of high immune power with other obvious advantages. Besides this they would assist in vitamin and essential nutrient synthesis that are required for your health. The diversified microbes in your gut would reduce the anxiety and depressions and make us feel good. The list doesn’t end here, it goes on.
……And I sat there imaging this conversation we could have in near future.
May be I would persuade him, maybe I’ll help him learn some cool vegan comebacks! Like, it saves animals, reduces global warming, or it’s the best way to be healthy and even make him talk about good bacteria - good mood...etc. Our world would be so perfect, with us chilling, taking pics with cute animals; would look amazing wearing those animal rights tees and of course, shopping for our vegan diet!!

[](/media/uploads/blog/58b.jpg)
[

](/media/uploads/blog/58b.jpg)
**Everything is fair in love isn’t it** **?**
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---
## Exasperation around discovery of new antibiotics
Author: BugSpeaks
Published: 2015-05-28
Category: Others
Meta Title: Exasperation around discovery of new antibiotics
Meta Description: It is exasperating to notice the continued discovery and use of antibiotics, even after the world has proclaimed “the golden age of antibiotics
URL: https://www.bugspeaks.com/blog/exasperation-around-discovery-of-new-antibiotics
It is exasperating to notice the continued discovery and use of antibiotics, even after the world has proclaimed “[the golden age of antibiotics to be over](http://s.telegraph.co.uk/graphics/projects/antibiotic-resistance/)” and that we have reached the brim of post-antibiotic era, after witnessing the unprecedented rise in the antimicrobial resistance. To add to the woes, the international agenda steering infectious disease control, intervention and elimination (is still) defined around “discovery of new antibiotics”, as if, the control and prevention of [already abundant count of resistant pathogens](http://www.cdc.gov/drugresistance/threat-report-2013/) is not a big ask.

[](/media/uploads/blog/6a.jpg)
[

](/media/uploads/blog/6a.jpg)
[Too much of a good thing, by Joe Shute](http://s.telegraph.co.uk/graphics/projects/antibiotic-resistance/), © Copyright of Telegraph Media Group Limited 2013
Current guidelines and strategies do recognize and support the attempts to decelerate the spread of existing resistant infections, emergence of new resistant pathogens through expansion & strengthening of surveillance efforts and development & deployment of rapid diagnostic tests. This shift away from just presumptive treatment towards properly diagnosed and directed treatments is welcome, but it does not justify the continued use of current antibiotics and certainly not the discovery of new ones.
On the contrary, over past few years, there has been a renewed interest in finding new antibiotics, with this particular research group claiming the discovery of “[a new game changing antibiotic](http://www.nature.com/nature/journal/v517/n7535/full/nature14098.html#affil-auth)”. Accordingly, “teixobactin”, mined from “[deep soil microbial dark matter](http://kemnovation.com/use-of-microbial-genomics-to-identify-soil-dark-matter/)”, is capable of combating MRSA (Methicillin-Resistant_Staphylococcus_ aureus) and multi-drug-resistant _Mycobacterium_ tuberculosis. The attribute that sets apart teixobactin from other antibiotics is “the indication that it will be difficult for pathogens to develop resistance against it”. “Unusually for an antibiotic, teixobactin is thought to attack microbes by binding to fatty lipids that make up the bacterial cell wall, and it is difficult for a bacterium to alter such fundamental building blocks of the cell. By comparison, most antibiotics target proteins and it can be relatively easy for a microbe to become resistant to those drugs by accumulating mutations that alter the target protein’s shape” [explains, Dr. Kim Lewis](http://www.nature.com/news/promising-antibiotic-discovered-in-microbial-dark-matter-1.16675#/b1). It is possible that certain antibiotics, based on their mode of action, might have a low frequency of resistance, however, it is improbable to perceive that microbes cannot develop resistance to it, if so, it will be (foolishly) similar to the original claim that “[the time has come to close the book on infectious diseases](http://www.who.int/bulletin/volumes/86/8/08-056242/en/)”.

[](/media/uploads/blog/6b.jpg)
[

](/media/uploads/blog/6b.jpg)
This progressively worsening issue of discovery, (over) use and resistance to drugs is expanding beyond bacterial pathogens, with the discovery of emerging parasite resistance to antimalarials and mosquito resistance to insecticides. Rise of multi drug resistant _Plasmodium_ falciparum has been globally reported, with [emerging parasite resistance to artemisinin](http://www.wellcome.ac.uk/News/Media-office/Press-releases/2015/WTP058719.htm), which is recognized to be an immediate threat to the recent progress and a major obstacle to the future control of malaria. Inanely, [the strategies employed for malaria intervention](http://blogs.plos.org/biologue/2015/04/23/stressed-to-death/) is no different than the contemporary case of bacterial pathogens, with approaches such as combinational therapy or extended therapy (extending the treatment from 3 to 6 days). Another approach that has caught some attention, is treating drug-resistant parasites with [artemisinins and a proteasome inhibitor](http://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.1002132), which in combination has the ability to stress the parasite and prevent its ability to protect itself from damaged proteins, hence synergistically killing the resistant parasites. The use of proteasome inhibitor has been around since early 2000, and has been [successfully tested for cancer therapy](http://www.nlm.nih.gov/medlineplus/druginfo/meds/a607007.html) before. However, its use in combination with artemisinin to combat drug resistant malaria seems fatuous since the intrinsic and acquired resistance to proteasome inhibitor drugs like “bortezomib” has already been reported, with several [mechanisms being studied extensively](http://www.ncbi.nlm.nih.gov/pubmed/22978849).
There might be some utility for conserved and appropriate use of current (and new) drugs, say in case of emergencies or until suitable vaccines are developed against, but it is should not be a sustainable model for the future nor it should hinder the pursuit for safer alternative approaches. It is imperative to learn from our (bad) experience and presume that most of the current tools against these pathogens and even newly discovered drugs could render ineffective in near (rather than far) future. It is absolutely necessary to reiterate the fact that we are dealing with highly adaptive life forms and the only way to tackle them is to develop equally adaptive combative therapies, keeping in mind their evolutionary projectile.
The actual shift has to be “a cultural evolution to create an environment where we do not use or need antibiotics.” The dramatic progress that France made through the “[Antibiotics Are Not Automatic](http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000080)” campaign, can be a notable example that demonstrates the positive cumulative impact of reducing the overuse of antibiotic.
Advancement in molecular methods and genomics have enabled deeper comprehension of pathogen-host interactions, which in turn have paved way for new[approaches to develop non-chemical antimicrobial strategies](http://blogs.cdc.gov/genomics/2014/10/30/outsmarting/). Utilization and translation of decades of molecular studies identifying genetic variations involving drug resistance, virulence, transmission and human immunity, followed by completion of the whole genome sequences of pathogens, vectors and the human genome, should lead us to relevant clinical tools for intervention and elimination of these pathogens. Any limitations on the way should force us to concentrate our resources in areas where we can identify and exploit significant susceptibilities of these complex pathogens, instead of relying on “Einstein’s definition of Insanity”.
---
This blog is powered by Superblog. Visit https://superblog.ai to know more.
---