Holobiome: Research I Articles I Updates I News I Series I Feed
Holobiome is a blog series that offers an AI-assisted summary of the latest research articles on human microbiome.
Why Omega-3 Works Better for Some People—Your Microbiome May Hold the Answer
This study explored how omega-3 rich fish oil—loaded with the healthy fats DHA and EPA—affects both the fat molecules and gut bacteria in people with type 2 diabetes and elevated triglycerides. Over 12 weeks, patients were randomly assigned to take either 4 grams of fish oil or a corn oil placebo. As expected, fish oil led to a notably larger drop in blood triglycerides, roughly cutting them by about 1.5 mmol/L versus only 0.7 mmol/L in the corn oil group.
Looking closer at blood markers, researchers saw that fish oil shifted the lipid landscape by reducing triglyceride types with few unsaturated bonds, while boosting those rich in DHA or EPA. In other words, the fats in the bloodstream became more “omega-3-like” after supplementation.
Turning to the gut microbiome, the fish oil didn’t cause major upheavals in the overall microbial makeup—changes were subtle. But intriguing findings emerged when researchers analyzed who responded best: baseline differences in a handful of microbial features actually predicted how much someone’s triglycerides would drop. These microbiome signals were even better predictors of response than typical clinical traits or the lipidomic profile itself.
Furthermore, the team identified nine specific lipid molecules that seemed to serve as a bridge between those microbial markers and the triglyceride response. In essence, having the “right” gut bugs before supplementation helped determine how well the body responded—possibly via those lipid intermediaries. Although particular microbial species or strains weren’t named, this finding underscores a tantalizing insight: our gut microbial makeup may help explain why omega-3 fats work better in some people than others, pointing toward more personalized nutrition strategies.
Prebiotics in Later Life: How 2’-FL Supercharges Bifidobacterium and Health
A team of researchers recently asked whether 2’-fucosyllactose (2’-FL), a special sugar naturally found in human breast milk, could offer benefits in older adults—beyond its well-known advantages for infants. In a six-week randomized trial involving nearly 90 healthy folks averaging 67 years of age, participants took daily doses of 2’-FL. Although the study didn’t meet its main goal of improving immune-related cytokine responses, it revealed some exciting microbiome and metabolic shifts.
What really caught attention was how 2’-FL seemed to gently reshape the gut microbiome—particularly by boosting levels of Bifidobacterium, the friendly bacteria known to support digestion and well-being. But not everyone responded the same. Those who already had Bifidobacterium in their gut ("responders") saw extra metabolic advantages: improvements in serum insulin, higher HDL (“good”) cholesterol, and elevated levels of FGF21, a hormone that plays a role in energy balance.
Even more intriguing, the people in this study with Bifidobacterium not only benefited metabolically but also performed better on a visual memory test. In contrast, participants who initially lacked Bifidobacterium appeared less likely to reap these perks.
This trial highlights the promise of a simple prebiotic—2’-FL—in shifting the gut ecosystem toward healthier outcomes. It also underscores how having the "right" microbes up front can influence who benefits most. That hints at a future where personalized nutrition—based on the microbiome—helps support healthy aging.
From Dysbiosis to Defense: How a Tailored Synbiotic Calms the Vaginal Microbiome
A recent randomized, placebo-controlled study explored whether a specially designed vaginal synbiotic—housing multiple strains of Lactobacillus crispatus—could help restore a healthy vaginal microbiome in women whose communities were out of balance. Delivered directly to the vagina shortly after menstruation, this synbiotic succeeded in creating a microbiome dominated by L. crispatus—dubbed the ideal “CST I” state—in 90 % of women, compared to just 11 % with placebo.
This shift wasn’t just about increasing good bacteria. Women using the synbiotic showed significant reductions in classic culprits of vaginal dysbiosis—Gardnerella vaginalis and several Candida species (including C. albicans, C. tropicalis, and C. parapsilosis), microbes often linked to infections and discomfort. In lab tests, each L. crispatus strain—and especially the combined consortium—powerfully inhibited Gardnerella growth, reinforcing these in-human findings.
Beyond the microbial makeover, the synbiotic lowered the presence of sialidase genes—microbial enzymes that erode the mucus barrier protecting the vaginal lining—and reduced levels of the inflammatory signal IL-1α, hinting not only at microbial rebalancing but also strengthened physical defenses and calmer immune activity.
Together, these outcomes offer a compelling glimpse into how targeted bacterial therapy—using native, multi-strain probiotics—can guide the vaginal ecosystem back to health. Without antibiotics, women regained a protective microbiome and reduced harmful organisms. This study underscores the promise of precision microbiome interventions in women’s health, especially using thoughtfully composed live communities tailored to the body’s natural ecology.
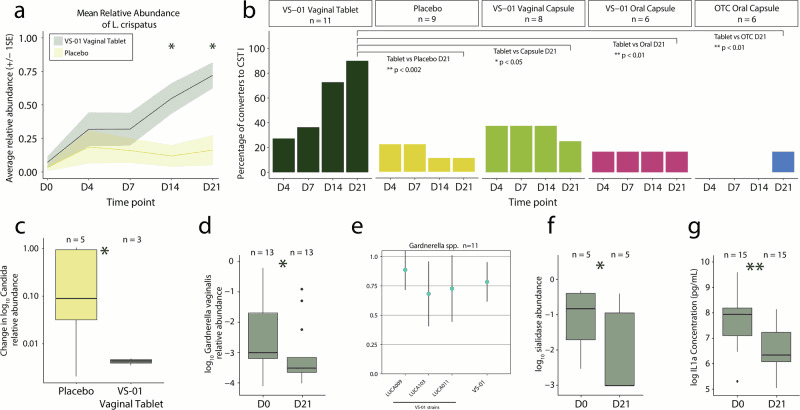
a) Mean relative abundance of L. crispatus in the multi-strain synbiotic vaginal tablet (VS-01) and placebo arms (n = 19) among participants not in CST I at baseline (<50% relative abundance of L. crispatus, as defined in Methods). * indicates p < 0.05 difference between the multi-strain synbiotic and placebo; b) The proportion of participants who converted to CST I at each timepoint is shown for Part A and Part B among those with a baseline dysbiotic/non-optimal microbiome (n = 39), along with associated p values; c VS-01 significantly decreased the mean abundance of Candida spp. (C. albicans,C. tropicalis, and C. parapsilosis) compared to placebo from baseline to D21 (p < 0.05); d VS-01 significantly decreased the abundance of Gardnerella vaginalis, a key dysbiotic/non-optimal bacterium, from baseline to D21 (p < 0.05); e Each strain in VS-01 and the VS-01 consortium inhibited the growth of Gardnerella spp. in vitro. The fraction of inhibition is shown across all strains, with 1 representing complete inhibition and 0 representing no inhibition; f VS-01 significantly decreased sialidase genes from baseline to D21 among participants with baseline detectable sialidase genes (p < 0.05); g VS-01 significantly decreased the concentration of IL-1ɑ from baseline to D21 (p < 0.01).
Gut Check: How One Bacterial Family Determines Fecal Transplant Results in UC
In this study, researchers explored how the gut’s microbial communities change over time in patients with ulcerative colitis (UC) after undergoing fecal microbiota transplantation (FMT). The key finding? Success in therapy wasn’t just about introducing new bacteria—it was about keeping a particular family of microbes, Prevotellaceae, under control. Patients whose condition improved showed that Prevotellaceae remained stabilized or reduced, hinting these microbes might play a role in flare-ups when they overgrow. Meanwhile, those who didn’t respond as well often experienced shifts involving this family. Tracking these dynamics over the course of treatment could help doctors gauge early on whether FMT is on the right track.
What makes this study compelling from a microbiome perspective is the focus on specific microbial families instead of just broad diversity shifts. Prevotellaceae has been linked in other research to inflammatory states, and here its behavior turns into a potential early warning signal—'if it breaks loose, the treatment may falter'. That level of insight points toward a more refined, microbe-level roadmap for monitoring FMT success.
These findings remind us that the gut microbiome isn't a static player; it’s a dynamic, living ecosystem where stability—or lack thereof—can make or break treatment outcomes. It also hints that future UC therapies might benefit from adjusting protocols based on how microbial families like Prevotellaceae behave in real time.
As research moves toward precision interventions, monitoring such microbial markers could guide personalized care—shaping when to adjust treatment, add microbial boosters, or explore alternative therapies before a relapse begins.
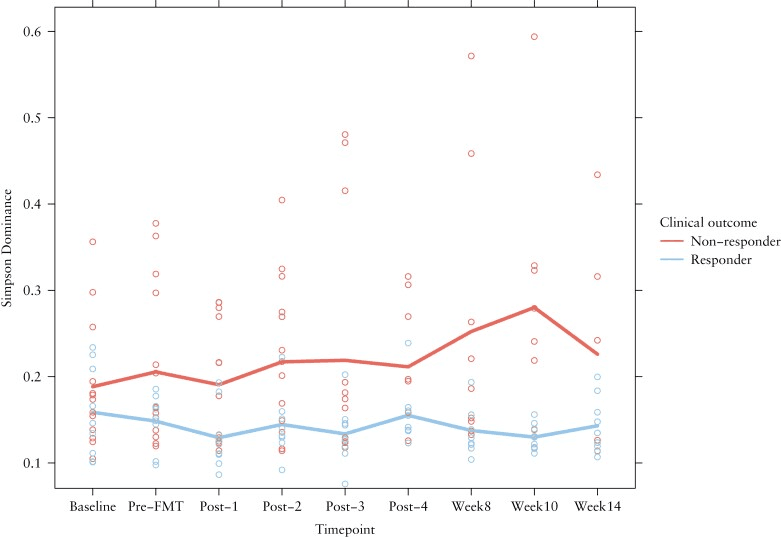
Change in Simpson dominance was calculated for non-responders and responders. The points indicate the individual measurements of the patients. The coloured lines are the mean Simpson dominance per group.
Taste Therapy via the Gut: Dried Miracle Berries and Microbial Boost in Cancer Patients
Many cancer patients undergoing treatment suffer from dysgeusia, a distortion or loss of taste, which can worsen malnutrition and quality of life. This study tested whether dried miracle berries (DMB)—rich in the taste-altering glycoprotein miraculin—could help when taken alongside standard nutritional care. In a carefully controlled trial, 31 malnourished patients were given either a standard dose (150 mg) or high dose (300 mg) of DMB tablets before each main meal, or a placebo, over three months. The researchers also analyzed patients’ gut microbiomes via stool samples using nanopore sequencing to see if the berries influenced microbial communities.
The findings revealed subtle but meaningful shifts in gut bacteria: both DMB groups saw changes in genera like Phocaeicola and Escherichia, while the standard-dose group showed greater levels of Solibaculum after three months. At the species level, Bacteroides sp. PHL 2737 dropped in both DMB groups, and Vescimonas coprocola rose in both treatment arms. These microbial tweaks were linked to immune and metabolic signals: the standard dose was tied to higher TNF-α levels and greater abundance of Lachnoclostridium and Mediterraneibacter, whereas the high dose was connected to lower TNF-α and reduced Phocaeicola.
On top of that, the high-dose group showed a positive relationship between erythrocyte polyunsaturated fatty acids (mindful markers of nutritional status) and beneficial genera such as Lachnoclostridium and Roseburia. Interestingly, Phocaeicola was linked to higher fecal acetic acid—but also, in high-dose users, to poorer taste perception. That hints at a nuanced interplay where the gut microbiome responds to DMB supplementation in ways that may support immune function, nutrition, and perhaps even taste recovery.
Though this was a small pilot trial, it opens a fascinating window into how a simple, taste-modifying food supplement might ease dysgeusia and malnutrition by gently nudging the gut microbiome. It suggests a future where targeted dietary tools help modulate microbial communities to support overall resilience in vulnerable patients.
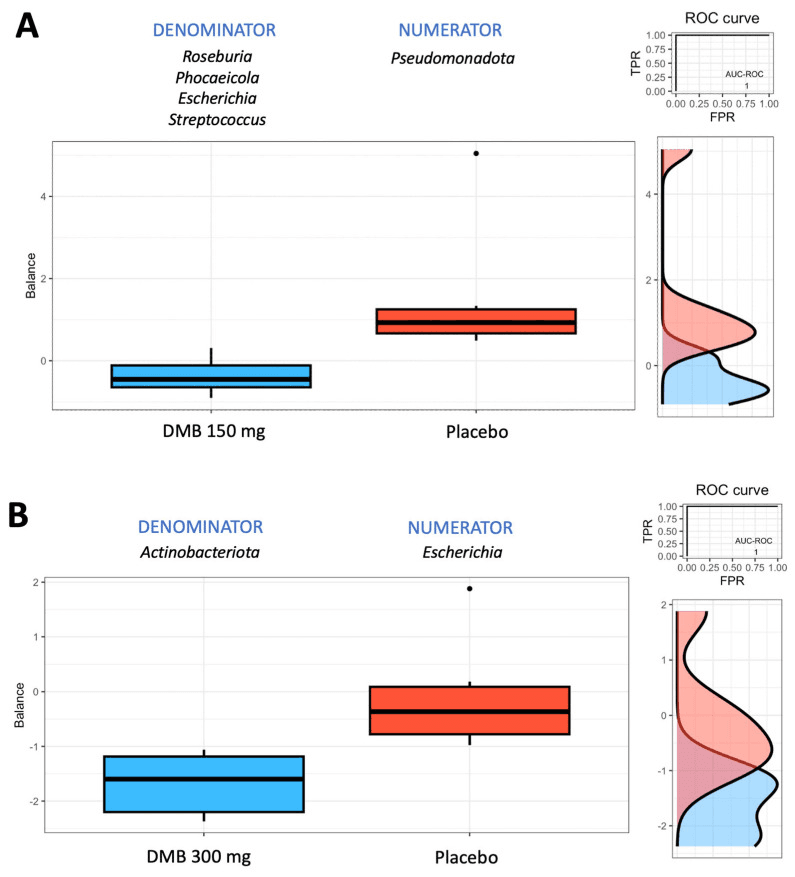
Group balances are presented in an overview. The top of the plot indicates that groups of taxa constitute the global balance. Box plots illustrating the distribution of balance scores for the DMB 150 mg (standard dose) and placebo groups (A) and the DMB 300 mg (high dose) and placebo groups (B). On the right, the ROC curve with its AUC value and the density curve are displayed.
