- Feb. 11, 2019
- BugSpeaks
- Microbiome and Disease
The gut microbiome promotes immune homeostasis
The gut microbiome is defined as the group of microorganisms and their genes existing inside the human gut. Recently, it has arisen as a primary factor in various human health conditions and diseases. A symbiotic relationship between humans and microbes is prevailing ever since from the birth, and any perturbations in this relationship could be the basis for immunological dysregulation, which might lead to the onset of many health complications and diseases including inflammatory bowel disease, autoimmunity, rheumatoid arthritis, cancer and multiple sclerosis. Symbiotic bacteria of the human gut provide a good environment and several advantages, such as providing essential nutrients, metabolizing indigestible food compounds, defense against the opportunistic pathogen colonization, and even contribute in the development of the intestinal architecture.
The immune system is responsible to recognize, retort and acclimatize to numerous foreign substances/molecules and also self-molecules. Thus, immune responses significantly modulate the development of health and disease. The human gut placidly co-exist with vast and diverse beneficial microbiota, and our immune system has evolved with gut bacteria to protect against various infectious microbial pathogens. In specific, the gastrointestinal tract is the main site at which both symbiotic and pathogenic microbes interact with the host immune system. More recent evidences have suggested a beneficial partnership has evolved amongst the symbiotic gut bacteria and the host immune system. The molecular communications/exchanges between them are directly linked to the growth of immune responses, and sequentially, the immune system of the host shapes the configuration of the microbiota.
Research evidences have related the role of the gut microbiota in shaping our immune system responses during diseases, however, the question still remains as to which specific bacteria are responsible in the mediation of these helpful responses and, more importantly, how this is accomplished. Some inspiring examples of the gut microorganisms having a pivotal role in the prevention of inflammatory bowel diseases, and beneficial immune reactions they stimulate during the defense are highlighted below.
At the start of 1900s, Ilya Mechnikov proposed for the first time about the use of live microbes to sustain bowel health and prolong life. Presently, the word “probiotic” is used for describing dietetic microbes with the ability of offering health benefits to the host. Numerous bacterial species either individually or in combination have been reported to ameliorate the symptoms of inflammatory bowel diseases in humans and mouse models. Some of them include Bifidobacteria lactis, B. infantis, E. coli Nissle 1917, Lactobacillus rhamnosus GG, L. salivarius, L. fermentum, Bacteriodes fragilis, B. thetaiotaomicron, etc. Mainly, these probiotic strains decrease toxic microbial metabolic events, however, more recent evidences have demonstrated their capability to modulate gut immune responses. The probiotic strains have a common feature of controlling the inflammation. The probiotic bacteria act on different cell types including epithelial cells, T cells and dendritic cells. Further, evidences have suggested that these probiotic strains induce regulatory T cells, which is the central to regulating inflammation and diseases. For example, treating colitic mice with the VSL#3 (a mixture of 8 lactic acid bacteria probiotic strains) increased the production of interleukin (IL-10) and the percentage of TGFβ-expressing T cells. Likewise, in another model of pathogen-induced inflammation study, the treatment of mice with B. infantis significantly down-regulated the intestinal inflammation and increased the number of CD4+CD25+ TReg cells. Moreover, the adaptive transmission of the CD4+CD25+ TReg-cells from mice served with B. infantis repressed inflammation-related activation of NF-κB (nuclear factor-κB) in the recipient mice. Interestingly, when the bone-marrow-derived dendritic cells incubated with L. rhamnosus were transferred into a colitic mice, a protection against inflammation and disease was observed. Further studies have shown that L. rhamnosus-treated dendritic cells can initiate TReg-cell activity. It has been shown that certain patients with Crohn's disease possess a reduced level of a noticeable gut bacteria, Faecalibacterium prausnitzii. As reviewed and explained by Round and Mazmanian (2009), this organism or its secreted substances are capable of inducing IL-10 expression (anti-inflammatory response), and ameliorate the induction of tumor necrosis factor-α (TNF-α) and bowel diseases, when administered orally to the experimental animals. This study further indicated that there exists a direct link between the reduced numbers of F. prausnitzii from the gut microbiota and the development of a bowel disease. This clearly indicates that symbiotic gut bacteria can intermediate in inflammatory bowel diseases and health. However, the explicit molecules/compounds secreted by these beneficial microbes of gut microbiota to guide in the immune responses still remains unclear. But, existing scientific data support the clue that symbiotic gut microbes actively interconnect with the host immune system to modulate anti-inflammatory processes.
A single molecule, polysaccharide A (PSA) secreted by the human commensal bacterium (Bacteroides fragilis) is predicted to shape favorable immune responses (Figure 1). When the germ-free mice were colonized with B. fragilis or treated with the purified PSA, the cellular and physical development of the immune system was improved with the increase of differentiated splenic CD4+ T cells. Experimental evidences have suggested that PSA protects by decreasing the levels of the pro-inflammatory cytokines (TNFα, IL-17 and IL-23). Also, it inhibits epithelial hyperplasia and neutrophil infiltration to the gut associated with disease induction in these models (Round and Mazmanian 2009). Likewise, the gut microbiota on T-cells immune responses are depicted in figure 1.
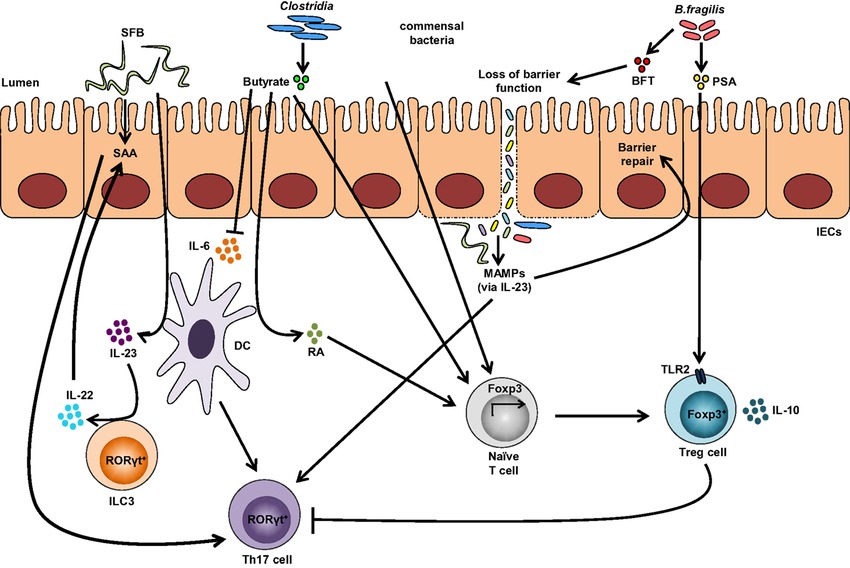
Figure-1: Impact of the gut microbiota on T-cells immune responses. Colonization with segmented filamentous bacteria (SFB) occurs by intimate attachment to the intestinal epithelium and promotes the development of T-helper 17 (Th17) cells via intestinal epithelial cell (IEC)-derived cytokines, serum amyloid A (SAA), as well as antigen presentation by dendritic cells (DCs). Adhesion of SFB to IEC can potentially generate a circuit, wherein DC-derived IL-23 stimulates IL-22 production by type 3 innate lymphoid cells (ILC3), which in turn induces SAA from IEC and can lead to Th17 cell differentiation. Conversely, colonization of beneficial commensal bacteria induces de novo generation of Tregs and downregulates Th17 immune responses. Commensal bacteria, including most Clostridia species, produce short-chain fatty acids, i.e., butyrate, which participates in the de novo generation of T-regulatory cells by suppressing proinflammatory cytokines, by promoting RA production from DCs, and by inducing Foxp3 transcription. Among different Bacteroides fragilis strains, those expressing polysaccharide A (PSA) mediate the generation of Tregs via TLR2, while those secreting B. fragilis toxin (BFT) alter the function of IEC tight junctions. Upon disruption of barrier function, dissemination of microbial products, recognized by microbe-associated molecular patterns (MAMPs), occurs and activates the IL-23 pathway, resulting in subsequent barrier repair and stimulation of Th17 immune responses. (Adapted from Omenetti et al. 2015;https://doi.org/10.3389/fimmu.2015.00639).
Nevertheless, it can be predicted that some beneficial symbiont microbial species have evolved, and produce molecules that can prompt defensive intestinal immune reactions. The presently available treatments for inflammatory bowel diseases are either ineffectual in most patients or result in severe side effects. Therefore, understanding of the beneficial gut microbial species and their secreted compounds can aid in preventing or curing such diseases, and help in designing new and effective natural therapeutics against several inflammatory bowel diseases in near future.
References:
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009 May;9(5):313.
- Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Frontiers in immunology. 2015 Dec 17;6:639.